RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-alpha and NF-kappaB
- PMID: 15699076
- PMCID: PMC2213026
- DOI: 10.1084/jem.20040934
RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-alpha and NF-kappaB
Abstract
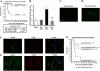
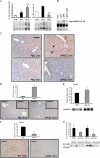
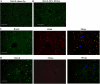
The exquisite ability of the liver to regenerate is finite. Identification of mechanisms that limit regeneration after massive injury holds the key to expanding the limits of liver transplantation and salvaging livers and hosts overwhelmed by carcinoma and toxic insults. Receptor for advanced glycation endproducts (RAGE) is up-regulated in liver remnants selectively after massive (85%) versus partial (70%) hepatectomy, principally in mononuclear phagocyte-derived dendritic cells (MPDDCs). Blockade of RAGE, using pharmacological antagonists or transgenic mice in which a signaling-deficient RAGE mutant is expressed in cells of mononuclear phagocyte lineage, significantly increases survival after massive liver resection. In the first hours after massive resection, remnants retrieved from RAGE-blocked mice displayed increased activated NF-kappaB, principally in hepatocytes, and enhanced expression of regeneration-promoting cytokines, TNF-alpha and IL-6, and the antiinflammatory cytokine, IL-10. Hepatocyte proliferation was increased by RAGE blockade, in parallel with significantly reduced apoptosis. These data highlight central roles for RAGE and MPDDCs in modulation of cell death-promoting mechanisms in massive hepatectomy and suggest that RAGE blockade is a novel strategy to promote regeneration in the massively injured liver.
Figures

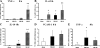

Similar articles
-
Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice.Hepatology. 2004 Feb;39(2):422-32. doi: 10.1002/hep.20045. Hepatology. 2004. PMID: 14767995
-
Receptor for advanced glycation end product (RAGE)-dependent modulation of early growth response-1 in hepatic ischemia/reperfusion injury.J Hepatol. 2009 May;50(5):929-36. doi: 10.1016/j.jhep.2008.11.022. Epub 2009 Jan 14. J Hepatol. 2009. PMID: 19303658
-
Loss of NF-kappaB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy.Am J Physiol Gastrointest Liver Physiol. 2007 Jun;292(6):G1570-7. doi: 10.1152/ajpgi.00399.2006. Epub 2007 Feb 22. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17322066
-
Hepatic tumor necrosis factor signaling and nuclear factor-kappaB: effects on liver homeostasis and beyond.Endocr Rev. 2007 Jun;28(4):365-86. doi: 10.1210/er.2006-0031. Epub 2007 Apr 12. Endocr Rev. 2007. PMID: 17431229 Review.
-
Cytokine regulation of liver injury and repair.Immunol Rev. 2000 Apr;174:160-71. doi: 10.1034/j.1600-0528.2002.017411.x. Immunol Rev. 2000. PMID: 10807515 Review.
Cited by
-
The ligand/RAGE axis: lighting the fuse and igniting vascular stress.Curr Atheroscler Rep. 2006 May;8(3):232-9. doi: 10.1007/s11883-006-0078-9. Curr Atheroscler Rep. 2006. PMID: 16640960 Review.
-
RAGE-specific single chain Fv for PET imaging of pancreatic cancer.PLoS One. 2018 Mar 12;13(3):e0192821. doi: 10.1371/journal.pone.0192821. eCollection 2018. PLoS One. 2018. PMID: 29529089 Free PMC article.
-
Follicular fluid soluble receptor for advanced glycation endproducts (sRAGE): a potential protective role in polycystic ovary syndrome.J Assist Reprod Genet. 2016 Jul;33(7):959-65. doi: 10.1007/s10815-016-0704-6. Epub 2016 Mar 24. J Assist Reprod Genet. 2016. PMID: 27011370 Free PMC article.
-
RAGE and the pathogenesis of chronic kidney disease.Nat Rev Nephrol. 2010 Jun;6(6):352-60. doi: 10.1038/nrneph.2010.54. Epub 2010 Apr 27. Nat Rev Nephrol. 2010. PMID: 20421886 Review.
-
Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice.J Clin Invest. 2008 Jan;118(1):183-94. doi: 10.1172/JCI32703. J Clin Invest. 2008. PMID: 18079965 Free PMC article.
References
-
- Fausto, N. 2000. Liver regeneration. J. Hepatol. 32:19–31. - PubMed
-
- Schulte-Hermann, R., B. Grasl-Kraupp, and W. Bursch. 1995. Apoptosis and hepatocarcinogenesis. In Liver Regeneration and Carcinogenesis. Molecular and Cellular Mechanisms. R.L. Jirtle, editor. Academic Press, San Diego, CA. 141–178.
-
- Panis, Y., D.M. McMullan, and J.C. Emond. 1997. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 121:142–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

