Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909
- PMID: 15696196
- PMCID: PMC546459
- DOI: 10.1172/JCI23373
Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909
Abstract
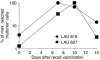
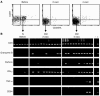
The induction of potent CD8+ T cell responses by vaccines to fight microbes or tumors remains a major challenge, as many candidates for human vaccines have proved to be poorly immunogenic. Deoxycytidyl-deoxyguanosin oligodeoxynucleotides (CpG ODNs) trigger Toll-like receptor 9, resulting in dendritic cell maturation that can enhance immunogenicity of peptide-based vaccines in mice. We tested whether a synthetic ODN, CpG 7909, could improve human tumor antigen-specific CD8+ T cell responses. Eight HLA-A2+ melanoma patients received 4 monthly vaccinations of low-dose CpG 7909 mixed with melanoma antigen A (Melan-A; identical to MART-1) analog peptide and incomplete Freund's adjuvant. All patients exhibited rapid and strong antigen-specific T cell responses: the frequency of Melan-A-specific T cells reached over 3% of circulating CD8+ T cells. This was one order of magnitude higher than the frequency seen in 8 control patients treated similarly but without CpG and 1-3 orders of magnitude higher than that seen in previous studies with synthetic vaccines. The enhanced T cell populations consisted primarily of effector memory cells, which in part secreted IFN- and expressed granzyme B and perforin ex vivo. In vitro, T cell clones recognized and killed melanoma cells in an antigen-specific manner. Thus, CpG 7909 is an efficient vaccine adjuvant that promotes strong antigen-specific CD8+ T cell responses in humans.
Figures

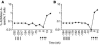

Similar articles
-
Simultaneous CD8+ T cell responses to multiple tumor antigen epitopes in a multipeptide melanoma vaccine.Cancer Immun. 2003 Oct 28;3:15. Cancer Immun. 2003. PMID: 14580186
-
Rapid and Continued T-Cell Differentiation into Long-term Effector and Memory Stem Cells in Vaccinated Melanoma Patients.Clin Cancer Res. 2017 Jul 1;23(13):3285-3296. doi: 10.1158/1078-0432.CCR-16-1708. Epub 2016 Nov 21. Clin Cancer Res. 2017. PMID: 27872103
-
Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8⁺ T-cell responses in melanoma patients.Eur J Immunol. 2012 Nov;42(11):3049-61. doi: 10.1002/eji.201142361. Epub 2012 Aug 28. Eur J Immunol. 2012. PMID: 22806397 Free PMC article. Clinical Trial.
-
The adjuvant effect of melanin is superior to incomplete Freund's adjuvant in subunit/peptide vaccines in mice.Cancer Immunol Immunother. 2020 Dec;69(12):2501-2512. doi: 10.1007/s00262-020-02631-7. Epub 2020 Jun 19. Cancer Immunol Immunother. 2020. PMID: 32561966 Free PMC article.
-
Ex vivo detectable activation of Melan-A-specific T cells correlating with inflammatory skin reactions in melanoma patients vaccinated with peptides in IFA.Cancer Immun. 2004 May 19;4:4. Cancer Immun. 2004. PMID: 15149168 Clinical Trial.
Cited by
-
Interrogating the recognition landscape of a conserved HIV-specific TCR reveals distinct bacterial peptide cross-reactivity.Elife. 2020 Jul 27;9:e58128. doi: 10.7554/eLife.58128. Elife. 2020. PMID: 32716298 Free PMC article.
-
X-shaped DNA potentiates therapeutic efficacy in colitis-associated colon cancer through dual activation of TLR9 and inflammasomes.Mol Cancer. 2015 May 15;14:104. doi: 10.1186/s12943-015-0369-2. Mol Cancer. 2015. PMID: 25971982 Free PMC article.
-
Toll-like receptors in tumor immunotherapy.Clin Cancer Res. 2007 Sep 15;13(18 Pt 1):5280-9. doi: 10.1158/1078-0432.CCR-07-1378. Clin Cancer Res. 2007. PMID: 17875756 Free PMC article. Review.
-
MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model.Vaccine. 2007 Feb 19;25(9):1607-18. doi: 10.1016/j.vaccine.2006.11.007. Epub 2006 Nov 16. Vaccine. 2007. PMID: 17166639 Free PMC article.
-
High-dose RHAMM-R3 peptide vaccination for patients with acute myeloid leukemia, myelodysplastic syndrome and multiple myeloma.Haematologica. 2010 Jul;95(7):1191-7. doi: 10.3324/haematol.2009.014704. Epub 2010 Jan 15. Haematologica. 2010. PMID: 20081055 Free PMC article.
References
-
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54:1–13. - PubMed
-
- Marrack P, Kappler J. Subversion of the immune system by pathogens. Cell. 1994;76:323–332. - PubMed
-
- Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 1997;9:4–9. - PubMed
-
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2001;2:675–680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

