Insulin expressing hepatocytes not destroyed in transgenic NOD mice
- PMID: 15679918
- PMCID: PMC544947
- DOI: 10.1186/1740-2557-1-3
Insulin expressing hepatocytes not destroyed in transgenic NOD mice
Abstract
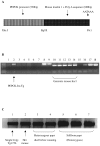
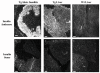
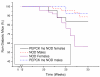
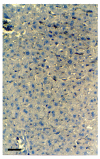
BACKGROUND: The liver has been suggested as a suitable target organ for gene therapy of Type 1 diabetes. However, the fundamental issue whether insulin-secreting hepatocytes in vivo will be destroyed by the autoimmune processes that kill pancreatic beta cells has not been fully addressed. It is possible that the insulin secreting liver cells will be destroyed by the immune system because hepatocytes express major histocompatibility complex (MHC) class I molecules and exhibit constitutive Fas expression; moreover the liver has antigen presenting activity. Together with previous reports that proinsulin is a possible autoantigen in the development of Type 1 diabetes, the autoimmune destruction of insulin producing liver cells is a distinct possibility. METHODS: To address this question, transgenic Non-Obese Diabetic (NOD) mice which express insulin in the liver were made using the Phosphoenolpyruvate Carboxykinase (PEPCK) promoter to drive the mouse insulin I gene (Ins). RESULTS: The liver cells were found to possess preproinsulin mRNA, translate (pro)insulin in vivo and release it when exposed to 100 nmol/l glucagon in vitro. The amount of insulin produced was however significantly lower than that produced by the pancreas. The transgenic PEPCK-Ins NOD mice became diabetic at 20-25 weeks of age, with blood glucose levels of 24.1 +/- 1.7 mmol/l. Haematoxylin and eosin staining of liver sections from these transgenic NOD PEPCK-Ins mice revealed the absence of an infiltrate of immune cells, a feature that characterised the pancreatic islets of these mice. CONCLUSIONS: These data show that hepatocytes induced to produce (pro)insulin in NOD mice are not destroyed by an ongoing autoimmune response; furthermore the expression of (pro)insulin in hepatocytes is insufficient to prevent development of diabetes in NOD mice. These results support the use of liver cells as a potential therapy for type 1 diabetes. However it is possible that a certain threshold level of (pro)insulin production might have to be reached to trigger the autoimmune response.
Figures


Similar articles
-
Insulin-producing intestinal K cells protect nonobese diabetic mice from autoimmune diabetes.Gastroenterology. 2014 Jul;147(1):162-171.e6. doi: 10.1053/j.gastro.2014.03.020. Epub 2014 Mar 21. Gastroenterology. 2014. PMID: 24662331
-
Effect on insulin production sorting and secretion by major histocompatibility complex class II gene expression in the pancreatic beta-cell of transgenic mice.Endocrinology. 1992 Aug;131(2):933-8. doi: 10.1210/endo.131.2.1639031. Endocrinology. 1992. PMID: 1639031
-
Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice.Diabetes. 1997 Jan;46(1):34-9. doi: 10.2337/diab.46.1.34. Diabetes. 1997. PMID: 8971078
-
Cellular and molecular roles of beta cell autoantigens, macrophages and T cells in the pathogenesis of autoimmune diabetes.Arch Pharm Res. 1999 Oct;22(5):437-47. doi: 10.1007/BF02979150. Arch Pharm Res. 1999. PMID: 10549569 Review.
-
Autoimmune diabetes: the role of T cells, MHC molecules and autoantigens.Autoimmunity. 1998;27(3):159-77. doi: 10.3109/08916939809003864. Autoimmunity. 1998. PMID: 9609134 Review.
Cited by
-
Glucose-regulated insulin production in the liver improves glycemic control in type 1 diabetic mice.Mol Metab. 2014 Nov 1;4(1):70-6. doi: 10.1016/j.molmet.2014.10.005. eCollection 2015 Jan. Mol Metab. 2014. PMID: 25685692 Free PMC article.
-
Failure to achieve normal metabolic response in non-obese diabetic mice and streptozotocin-induced diabetic mice after transplantation of primary murine hepatocytes electroporated with the human proinsulin gene (p3MTChins).Transplant Proc. 2014 Jul-Aug;46(6):2002-6. doi: 10.1016/j.transproceed.2014.05.068. Transplant Proc. 2014. PMID: 25131094 Free PMC article.
-
Remission of diabetes by insulin gene therapy using a hepatocyte-specific and glucose-responsive synthetic promoter.Mol Ther. 2011 Mar;19(3):470-8. doi: 10.1038/mt.2010.255. Epub 2010 Nov 30. Mol Ther. 2011. PMID: 21119621 Free PMC article.
-
Nonvirally modified autologous primary hepatocytes correct diabetes and prevent target organ injury in a large preclinical model.PLoS One. 2008 Mar 5;3(3):e1734. doi: 10.1371/journal.pone.0001734. PLoS One. 2008. PMID: 18320053 Free PMC article.
-
Pancreatic Transdifferentiation Using β-Cell Transcription Factors for Type 1 Diabetes Treatment.Cells. 2022 Jul 8;11(14):2145. doi: 10.3390/cells11142145. Cells. 2022. PMID: 35883588 Free PMC article. Review.
References
-
- Vollenweider F, Irminger JC, Gross DJ, Villa-Korniaroff L, Halban PA. Processing of proinsulin by transfected hepatoma (FAO) cells. J Biol Chem. 1992;267:14629–14636. - PubMed
-
- Gros L, Montoliu L, Riu E, Lebrigand L, Bosch F. Regulated production of mature insulin by non-β-cells. Hum Gene Ther. 1997;8:2249–2259. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

