DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures
- PMID: 15671176
- PMCID: PMC547890
- DOI: 10.1073/pnas.0409648102
DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures
Abstract
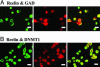
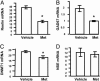
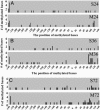
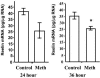
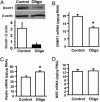
The polygenic nature of complex psychiatric disorders suggests a common pathway that may be involved in the down-regulation of multiple genes through an epigenetic mechanism. To investigate the role of methylation in down-regulating the expression of mRNAs that may be associated with the schizophrenia phenotype, we have adopted a cell-culture model amenable to this line of investigation. We have administered methionine (2 mM) to primary cultures of cortical neurons prepared from embryonic day 16 mice and show that this treatment down-regulated reelin and glutamic acid decarboxylase 67 (GAD67) mRNA expression but not that corresponding to neuron-specific enolase mRNA. Moreover, methionine increased methylation of the reelin promoter, suggesting a possible mechanism for the observed change. These cultures contain a mixed population of neurons and glia. Approximately 83% of the neurons are GABAergic based on GAD immunoreactivity, and these neurons coexpress high levels of reelin and DNA methyltransferase (Dnmt) 1 immunoreactivity. To examine whether Dnmt1 regulates reelin gene expression, we used an antisense approach to reduce (knock down) Dnmt1 expression. The reduced Dnmt1 mRNA and protein were accompanied by increased reelin mRNA expression. More importantly, the Dnmt1 knockdown blocked the methionine-induced reelin and GAD67 mRNA down-regulation. These data support the hypothesis that the reduced amounts of reelin and GAD67 mRNAs documented in postmortem schizophrenia brain may be the consequence of a Dnmt1-mediated hypermethylation of the corresponding promoters.
Figures
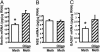
Similar articles
-
Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia.Proc Natl Acad Sci U S A. 2005 Aug 30;102(35):12578-83. doi: 10.1073/pnas.0505394102. Epub 2005 Aug 19. Proc Natl Acad Sci U S A. 2005. PMID: 16113080 Free PMC article.
-
The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes.Mol Pharmacol. 2009 Feb;75(2):342-54. doi: 10.1124/mol.108.051763. Epub 2008 Nov 24. Mol Pharmacol. 2009. PMID: 19029285 Free PMC article.
-
DNA methyltransferase inhibitors coordinately induce expression of the human reelin and glutamic acid decarboxylase 67 genes.Mol Pharmacol. 2007 Mar;71(3):644-53. doi: 10.1124/mol.106.030635. Epub 2006 Oct 25. Mol Pharmacol. 2007. PMID: 17065238
-
The human reelin gene: transcription factors (+), repressors (-) and the methylation switch (+/-) in schizophrenia.Pharmacol Ther. 2006 Jul;111(1):272-86. doi: 10.1016/j.pharmthera.2005.01.007. Epub 2006 Mar 30. Pharmacol Ther. 2006. PMID: 16574235 Review.
-
GABAergic cortical neuron chromatin as a putative target to treat schizophrenia vulnerability.Crit Rev Neurobiol. 2003;15(2):121-42. doi: 10.1615/critrevneurobiol.v15.i2.20. Crit Rev Neurobiol. 2003. PMID: 14977367 Review.
Cited by
-
RAS signalling through PI3-Kinase controls cell migration via modulation of Reelin expression.Nat Commun. 2016 Apr 13;7:11245. doi: 10.1038/ncomms11245. Nat Commun. 2016. PMID: 27071537 Free PMC article.
-
Histone deactylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: analysis of the National Brain Databank microarray collection.Schizophr Res. 2008 Jan;98(1-3):111-7. doi: 10.1016/j.schres.2007.09.020. Epub 2007 Oct 24. Schizophr Res. 2008. PMID: 17961987 Free PMC article.
-
Chromatin, DNA methylation and neuron gene regulation--the purpose of the package.J Psychiatry Neurosci. 2005 Jul;30(4):257-63. J Psychiatry Neurosci. 2005. PMID: 16049569 Free PMC article. Review.
-
The site specific demethylation in the 5'-regulatory area of NMDA receptor 2B subunit gene associated with CIE-induced up-regulation of transcription.PLoS One. 2010 Jan 20;5(1):e8798. doi: 10.1371/journal.pone.0008798. PLoS One. 2010. PMID: 20098704 Free PMC article.
-
Epigenetic RELN Dysfunction in Schizophrenia and Related Neuropsychiatric Disorders.Front Cell Neurosci. 2016 Apr 5;10:89. doi: 10.3389/fncel.2016.00089. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27092053 Free PMC article. Review.
References
-
- Petronis, A. (2001) Trends Genet. 17, 142–146. - PubMed
-
- Costa, E., Grayson, D. R., Mitchell, C. P., Tremolizzo, L., Veldic, M. & Guidotti, A. (2003) Crit. Rev. Neurobiol. 15, 121–142. - PubMed
-
- Robertson, K. D. (2001) Nat. Rev. Genet. 1, 11–19. - PubMed
-
- Tsujita, T., Niikawa, N., Yamashita, H., Imamura, A., Hamada, A., Nakane, Y. & Okazaki, Y. (1998) Am. J. Psychiatry 155, 422–424. - PubMed
-
- Petronis, A., Gottesman, I. I., Kan, P., Kennedy, J. L., Basile, V. S., Paterson, A. D. & Popendikyte, V. (2003) Schizophr. Bull. 29, 169–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

