Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin
- PMID: 15659595
- PMCID: PMC2275318
- DOI: 10.1523/JNEUROSCI.4011-04.2005
Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin
Abstract
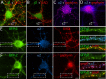
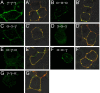
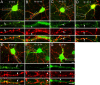
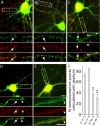
Modulation of the concentration of postsynaptic GABA(A) receptors contributes to functional plasticity of inhibitory synapses. The gamma2 subunit of GABA(A) receptor is specifically required for clustering of these receptors, for recruitment of the submembrane scaffold protein gephyrin to postsynaptic sites, and for postsynaptic function of GABAergic inhibitory synapses. To elucidate this mechanism, we here have mapped the gamma2 subunit domains required for restoration of postsynaptic clustering and function of GABA(A) receptors in gamma2 subunit mutant neurons. Transfection of gamma2-/- neurons with the gamma2 subunit but not the alpha2 subunit rescues postsynaptic clustering of GABA(A) receptors, results in recruitment of gephyrin to postsynaptic sites, and restores the amplitude and frequency of miniature inhibitory postsynaptic currents to wild-type levels. Analogous analyses of chimeric gamma2/alpha2 subunit constructs indicate, unexpectedly, that the fourth transmembrane domain of the gamma2 subunit is required and sufficient for postsynaptic clustering of GABA(A) receptors, whereas cytoplasmic gamma2 subunit domains are dispensable. In contrast, both the major cytoplasmic loop and the fourth transmembrane domain of the gamma2 subunit contribute to efficient recruitment of gephyrin to postsynaptic receptor clusters and are essential for restoration of miniature IPSCs. Our study points to a novel mechanism involved in targeting of GABA(A) receptors and gephyrin to inhibitory synapses.
Figures





Similar articles
-
Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice.J Neurosci. 1999 Nov 1;19(21):9289-97. doi: 10.1523/JNEUROSCI.19-21-09289.1999. J Neurosci. 1999. PMID: 10531433 Free PMC article.
-
Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons.J Neurosci. 2004 Jan 7;24(1):207-17. doi: 10.1523/JNEUROSCI.1661-03.2004. J Neurosci. 2004. PMID: 14715953 Free PMC article.
-
Distinct mechanisms regulate GABAA receptor and gephyrin clustering at perisomatic and axo-axonic synapses on CA1 pyramidal cells.J Physiol. 2011 Oct 15;589(Pt 20):4959-80. doi: 10.1113/jphysiol.2011.216028. Epub 2011 Aug 8. J Physiol. 2011. PMID: 21825022 Free PMC article.
-
Mini-review: gephyrin, a major postsynaptic protein of GABAergic synapses.Eur J Neurosci. 2000 Jul;12(7):2205-10. doi: 10.1046/j.1460-9568.2000.00106.x. Eur J Neurosci. 2000. PMID: 10947798 Review.
-
Molecular and functional heterogeneity of GABAergic synapses.Cell Mol Life Sci. 2012 Aug;69(15):2485-99. doi: 10.1007/s00018-012-0926-4. Cell Mol Life Sci. 2012. PMID: 22314501 Free PMC article. Review.
Cited by
-
Altered GABAA receptor expression in brainstem nuclei and SUDEP in Gabrg2(+/Q390X) mice associated with epileptic encephalopathy.Epilepsy Res. 2016 Jul;123:50-4. doi: 10.1016/j.eplepsyres.2016.04.002. Epub 2016 Apr 13. Epilepsy Res. 2016. PMID: 27131289 Free PMC article.
-
Bidirectional Homeostatic Regulation of a Depression-Related Brain State by Gamma-Aminobutyric Acidergic Deficits and Ketamine Treatment.Biol Psychiatry. 2016 Sep 15;80(6):457-468. doi: 10.1016/j.biopsych.2016.02.009. Epub 2016 Feb 13. Biol Psychiatry. 2016. PMID: 27062563 Free PMC article.
-
The residence time of GABA(A)Rs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin.J Neurosci. 2011 Oct 12;31(41):14677-87. doi: 10.1523/JNEUROSCI.2001-11.2011. J Neurosci. 2011. PMID: 21994384 Free PMC article.
-
Neuroligin 2 controls the maturation of GABAergic synapses and information processing in the retina.J Neurosci. 2009 Jun 24;29(25):8039-50. doi: 10.1523/JNEUROSCI.0534-09.2009. J Neurosci. 2009. PMID: 19553444 Free PMC article.
-
A versatile optical tool for studying synaptic GABAA receptor trafficking.J Cell Sci. 2017 Nov 15;130(22):3933-3945. doi: 10.1242/jcs.205286. Epub 2017 Oct 12. J Cell Sci. 2017. PMID: 29025969 Free PMC article.
References
-
- Banker G, Goslin K (1998) Culturing nerve cells. Cambridge, MA: MIT.
-
- Benson JA, Low K, Keist R, Mohler H, Rudolph U (1998) Pharmacology of recombinant γ-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated α-subunits. FEBS Lett 431: 400-404. - PubMed
-
- Blanton MP, Cohen JB (1994) Identifying the lipid-protein interface of the Torpedo nicotinic acetylcholine receptor: secondary structure implications. Biochemistry 33: 2859-2872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
