XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs
- PMID: 15650747
- PMCID: PMC548652
- DOI: 10.1038/sj.emboj.7600544
XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs
Abstract
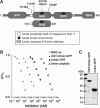
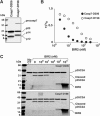
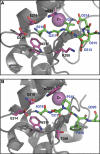
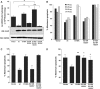
The X-linked inhibitor of apoptosis protein (XIAP) uses its second baculovirus IAP repeat domain (BIR2) to inhibit the apoptotic executioner caspase-3 and -7. Structural studies have demonstrated that it is not the BIR2 domain itself but a segment N-terminal to it that directly targets the activity of these caspases. These studies failed to demonstrate a role of the BIR2 domain in inhibition. We used site-directed mutagenesis of BIR2 and its linker to determine the mechanism of executioner caspase inhibition by XIAP. We show that the BIR2 domain contributes substantially to inhibition of executioner caspases. A surface groove on BIR2, which also binds to Smac/DIABLO, interacts with a neoepitope generated at the N-terminus of the caspase small subunit following activation. Therefore, BIR2 uses a two-site interaction mechanism to achieve high specificity and potency for inhibition. Moreover, for caspase-7, the precise location of the activating cleavage is critical for subsequent inhibition. Since apical caspases utilize this cleavage site differently, we predict that the origin of the death stimulus should dictate the efficiency of inhibition by XIAP.
Figures


Similar articles
-
A mechanistic insight into SMAC peptide interference with XIAP-Bir2 inhibition of executioner caspases.J Mol Biol. 2008 Sep 5;381(3):645-54. doi: 10.1016/j.jmb.2008.05.082. Epub 2008 Jun 7. J Mol Biol. 2008. PMID: 18619610
-
Engineering ML-IAP to produce an extraordinarily potent caspase 9 inhibitor: implications for Smac-dependent anti-apoptotic activity of ML-IAP.Biochem J. 2005 Jan 1;385(Pt 1):11-20. doi: 10.1042/BJ20041108. Biochem J. 2005. PMID: 15485396 Free PMC article.
-
A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis.Nature. 2001 Mar 1;410(6824):112-6. doi: 10.1038/35065125. Nature. 2001. PMID: 11242052
-
Mechanisms of caspase activation and inhibition during apoptosis.Mol Cell. 2002 Mar;9(3):459-70. doi: 10.1016/s1097-2765(02)00482-3. Mol Cell. 2002. PMID: 11931755 Review.
-
X-linked inhibitor of apoptosis protein - a critical death resistance regulator and therapeutic target for personalized cancer therapy.Front Oncol. 2014 Jul 28;4:197. doi: 10.3389/fonc.2014.00197. eCollection 2014. Front Oncol. 2014. PMID: 25120954 Free PMC article. Review.
Cited by
-
Phosphorylation regulates assembly of the caspase-6 substrate-binding groove.Structure. 2012 Apr 4;20(4):742-51. doi: 10.1016/j.str.2012.02.003. Epub 2012 Apr 3. Structure. 2012. PMID: 22483120 Free PMC article.
-
XAF1 promotes osteoclast apoptosis by antagonizing the XIAP-caspase axis.J Orthop Translat. 2024 Jun 7;47:15-28. doi: 10.1016/j.jot.2024.05.001. eCollection 2024 Jul. J Orthop Translat. 2024. PMID: 38957269 Free PMC article.
-
Modeling the effects of a Staphylococcal Enterotoxin B (SEB) on the apoptosis pathway.BMC Microbiol. 2006 May 31;6:48. doi: 10.1186/1471-2180-6-48. BMC Microbiol. 2006. PMID: 16737533 Free PMC article.
-
A small molecule inhibitor of XIAP induces apoptosis and synergises with vinorelbine and cisplatin in NSCLC.Br J Cancer. 2010 Jan 5;102(1):97-103. doi: 10.1038/sj.bjc.6605418. Epub 2009 Nov 10. Br J Cancer. 2010. PMID: 19904270 Free PMC article.
-
Contribution of caspase(s) to the cell cycle regulation at mitotic phase.PLoS One. 2011 Mar 30;6(3):e18449. doi: 10.1371/journal.pone.0018449. PLoS One. 2011. PMID: 21479177 Free PMC article.
References
-
- Barnhart BC, Peter ME (2002) Two faces of caspase-8. Nat Immunol 3: 896–898 - PubMed
-
- Bratton SB, Lewis J, Butterworth M, Duckett CS, Cohen GM (2002) XIAP inhibition of caspase-3 preserves its association with the Apaf-1 apoptosome and prevents CD95- and Bax-induced apoptosis. Cell Death Differ 9: 881–892 - PubMed
-
- Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y (2000) Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 406: 855–862 - PubMed
-
- Chai J, Shiozaki E, Srinivasula SM, Wu Q, Datta P, Alnemri ES, Shi Y, Dataa P (2001) Structural basis of caspase-7 inhibition by XIAP. Cell 104: 769–780 - PubMed
-
- Chai J, Yan N, Huh JR, Wu JW, Li W, Hay BA, Shi Y (2003) Molecular mechanism of Reaper–Grim–Hid-mediated suppression of DIAP1-dependent Dronc ubiquitination. Nat Struct Biol 10: 892–898 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

