Randomized, controlled trial of therapy interruption in chronic HIV-1 infection
- PMID: 15630469
- PMCID: PMC539050
- DOI: 10.1371/journal.pmed.0010064
Randomized, controlled trial of therapy interruption in chronic HIV-1 infection
Abstract
Background: Approaches to limiting exposure to antiretroviral therapy (ART) drugs are an active area of HIV therapy research. Here we present longitudinal follow-up of a randomized, open-label, single-center study of the immune, viral, and safety outcomes of structured therapy interruptions (TIs) in patients with chronically suppressed HIV-1 infection as compared to equal follow-up of patients on continuous therapy and including a final therapy interruption in both arms.
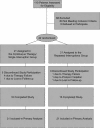
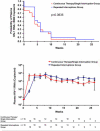
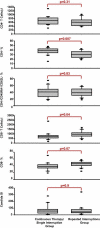
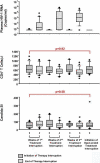
Methods and findings: Forty-two chronically HIV-infected patients on suppressive ART with CD4 counts higher than 400 were randomized 1:1 to either (1) three successive fixed TIs of 2, 4, and 6 wk, with intervening resumption of therapy with resuppression for 4 wk before subsequent interruption, or (2) 40 wk of continuous therapy, with a final open-ended TI in both treatment groups. Main outcome was analysis of the time to viral rebound (>5,000 copies/ml) during the open-ended TI. Secondary outcomes included study-defined safety criteria, viral resistance, therapy failure, and retention of immune reconstitution. There was no difference between the groups in time to viral rebound during the open-ended TI (continuous therapy/single TI, median [interquartile range] = 4 [1-8] wk, n = 21; repeated TI, median [interquartile range] = 5 [4-8] wk, n = 21; p = 0.36). No differences in study-related adverse events, viral set point at 12 or 20 wk of open-ended interruption, viral resistance or therapy failure, retention of CD4 T cell numbers on ART, or retention of lymphoproliferative recall antigen responses were noted between groups. Importantly, resistance detected shortly after initial viremia following the open-ended TI did not result in a lack of resuppression to less than 50 copies/ml after reinitiation of the same drug regimen.
Conclusion: Cycles of 2- to 6-wk time-fixed TIs in patients with suppressed HIV infection failed to confer a clinically significant benefit with regard to viral suppression off ART. Also, secondary analysis showed no difference between the two strategies in terms of safety, retention of immune reconstitution, and clinical therapy failure. Based on these findings, we suggest that further clinical research on the long-term consequences of TI strategies to decrease drug exposure is warranted.
Conflict of interest statement
RMG is a paid consultant for the Bayer Guidelines Project, which develops algorithms for interpretation of drug resistance genotyping assays; received honoraria and research support from ViroLogic and Visible Genetics; received honoraria for speaking at educational programs supported by ViroLogic, Visible Genetics, GlaxoSmithKline, Bristol-Myers Squibb, Roche Pharmaceuticals, and Agouron Pharmaceuticals; and directs a nonprofit academic laboratory at the Gladstone Institute of Virology and Immunology that has provided services for clinical research supported by grants to the University of California from Merck, GlaxoSmithKline, Bristol-Myers Squibb, Boehringer Ingelheim, Roche, Abbott, Agouron Pharmaceuticals, Gilead, Visible Genetics, and Chiron.
RG receives support for his HIV research from Agouron Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb and serves as a consultant to GlaxoSmithKline; all relationships have been disclosed to the University of Pennsylvania, which deemed them not to constitute a conflict of interest.
Figures

Similar articles
-
Structured treatment interruptions (STI) in chronic unsuppressed HIV infection in adults.Cochrane Database Syst Rev. 2006 Jul 19;2006(3):CD006148. doi: 10.1002/14651858.CD006148. Cochrane Database Syst Rev. 2006. PMID: 16856117 Free PMC article. Review.
-
A pilot study evaluating time to CD4 T-cell count <350 cells/mm(3) after treatment interruption following antiretroviral therapy +/- interleukin 2: results of ACTG A5102.J Acquir Immune Defic Syndr. 2006 Jun;42(2):140-8. doi: 10.1097/01.qai.0000225319.59652.1e. J Acquir Immune Defic Syndr. 2006. PMID: 16760795 Clinical Trial.
-
Cognitive functioning during highly active antiretroviral therapy interruption in human immunodeficiency virus type 1 infection.J Neurovirol. 2008 Nov;14(6):550-7. doi: 10.1080/13550280802372313. J Neurovirol. 2008. PMID: 19016380 Free PMC article.
-
Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression.AIDS. 2000 Mar 10;14(4):397-403. doi: 10.1097/00002030-200003100-00013. AIDS. 2000. PMID: 10770542
-
Structured treatment interruptions (STI) in chronic suppressed HIV infection in adults.Cochrane Database Syst Rev. 2005 Oct 19;(4):CD005482. doi: 10.1002/14651858.CD005482. Cochrane Database Syst Rev. 2005. PMID: 16235406 Review.
Cited by
-
Relationship of antiretroviral treatment to postmortem brain tissue viral load in human immunodeficiency virus-infected patients.J Neurovirol. 2006 Apr;12(2):100-7. doi: 10.1080/13550280600713932. J Neurovirol. 2006. PMID: 16798671
-
Immunological responses and long-term treatment interruption after human immunodeficiency virus type 1 (HIV-1) lipopeptide immunization of HIV-1-infected patients: the LIPTHERA study.Clin Vaccine Immunol. 2008 Mar;15(3):562-8. doi: 10.1128/CVI.00165-07. Epub 2008 Jan 9. Clin Vaccine Immunol. 2008. PMID: 18184824 Free PMC article. Clinical Trial.
-
Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells.PLoS Pathog. 2015 Nov 5;11(11):e1005233. doi: 10.1371/journal.ppat.1005233. eCollection 2015. PLoS Pathog. 2015. PMID: 26539983 Free PMC article.
-
Heightened resistance to host type 1 interferons characterizes HIV-1 at transmission and after antiretroviral therapy interruption.Sci Transl Med. 2021 Jan 13;13(576):eabd8179. doi: 10.1126/scitranslmed.abd8179. Sci Transl Med. 2021. PMID: 33441429 Free PMC article.
-
Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration.J Infect Dis. 2013 Jan 15;207(2):213-22. doi: 10.1093/infdis/jis663. Epub 2012 Oct 26. J Infect Dis. 2013. PMID: 23105144 Free PMC article. Clinical Trial.
References
-
- Yeni PG, Hammer SM, Hirsch MS, Saag MS, Schechter M, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004;292:251–265. - PubMed
-
- Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001;15:2109–2117. - PubMed
-
- Arya SC. Antiretroviral therapy in countries with low health expenditure. Lancet. 1998;351:1433–1434. - PubMed
-
- Stephenson J. AIDS researchers target poor adherence. JAMA. 1999;281:1069. - PubMed
-
- Bangsberg DR, Charlebois ED, Grant RM, Holodniy M, Deeks SG, et al. High levels of adherence do not prevent accumulation of HIV drug resistance mutations. AIDS. 2003;17:1925–1932. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

