Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase
- PMID: 15625103
- PMCID: PMC544320
- DOI: 10.1073/pnas.0408704102
Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase
Abstract
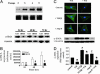
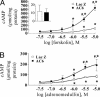
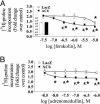
Transformation of fibroblasts to myofibroblasts, characterized by expression of alpha-smooth muscle actin (alpha-SMA) and production of extracellular matrix (ECM) components, is a key event in connective tissue remodeling. Approaches to inhibit this transformation are needed in tissues, such as the heart, where excessive ECM production by cardiac fibroblasts (CFs) causes fibrosis, myocardial stiffening, and cardiac dysfunction. We tested whether adenylyl cyclase (AC) activation (increased cAMP levels) modulates the transformation of adult rat CF to myofibroblasts, as assessed by immunofluorescent microscopy, immunoblotting, and collagen synthesis. A 24-h incubation of CF with TGF-beta or angiotensin II increased alpha-SMA expression, which was inhibited by the AC agonist forskolin and a cAMP analog that activates protein kinase A. Treatment with forskolin blunted serum-, TGF-beta-, and angiotensin II-stimulated collagen synthesis. CFs engineered to overexpress type 6 AC had enhanced forskolin-promoted cAMP formation, greater inhibition by forskolin of TGF-beta-stimulated alpha-SMA expression, and a decrease in the EC(50) of forskolin to reduce serum-stimulated collagen synthesis. The AC stimulatory agonist adrenomedullin inhibited collagen synthesis in CF that overexpressed AC6 but not in controls. Thus, AC stimulation blunts collagen synthesis and, in parallel, the transformation of adult rat CF to myofibroblasts. AC overexpression enhances these effects, "uncovering" an inhibition by adrenomedullin. These findings implicate cAMP as an inhibitor of ECM formation by means of blockade of the transformation of CF to myofibroblasts and suggest that increasing AC expression, thereby enhancing cAMP generation through stimulation of receptors expressed on CF, could provide a means to attenuate and prevent cardiac fibrosis and its sequelae.
Figures
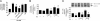
Similar articles
-
Increase in cellular cyclic AMP concentrations reverses the profibrogenic phenotype of cardiac myofibroblasts: a novel therapeutic approach for cardiac fibrosis.Mol Pharmacol. 2013 Dec;84(6):787-93. doi: 10.1124/mol.113.087742. Epub 2013 Oct 1. Mol Pharmacol. 2013. PMID: 24085841 Free PMC article.
-
cAMP inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts.Mol Pharmacol. 2006 Dec;70(6):1992-2003. doi: 10.1124/mol.106.028951. Epub 2006 Sep 7. Mol Pharmacol. 2006. PMID: 16959941
-
Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin. Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts.J Biol Chem. 2003 Jul 4;278(27):24461-8. doi: 10.1074/jbc.M212659200. Epub 2003 Apr 23. J Biol Chem. 2003. PMID: 12711600
-
Induction of cardiac fibrosis by transforming growth factor-beta(1).Mol Genet Metab. 2000 Sep-Oct;71(1-2):418-35. doi: 10.1006/mgme.2000.3032. Mol Genet Metab. 2000. PMID: 11001836 Review.
-
Functional properties of Ca2+-inhibitable type 5 and type 6 adenylyl cyclases and role of Ca2+ increase in the inhibition of intracellular cAMP content.Cell Signal. 1999 Sep;11(9):651-63. doi: 10.1016/s0898-6568(99)00031-5. Cell Signal. 1999. PMID: 10530873 Review.
Cited by
-
Adenylyl cyclase 6 deletion reduces left ventricular hypertrophy, dilation, dysfunction, and fibrosis in pressure-overloaded female mice.J Am Coll Cardiol. 2010 Apr 6;55(14):1476-86. doi: 10.1016/j.jacc.2009.11.066. J Am Coll Cardiol. 2010. PMID: 20359598 Free PMC article.
-
Regulation of cardiac fibroblast-mediated maladaptive ventricular remodeling by β-arrestins.PLoS One. 2019 Jul 3;14(7):e0219011. doi: 10.1371/journal.pone.0219011. eCollection 2019. PLoS One. 2019. PMID: 31269046 Free PMC article.
-
Biomarkers for the identification of cardiac fibroblast and myofibroblast cells.Heart Fail Rev. 2019 Jan;24(1):1-15. doi: 10.1007/s10741-018-9720-1. Heart Fail Rev. 2019. PMID: 29987445 Review.
-
Anti-fibrotic action of pirfenidone in Dupuytren's disease-derived fibroblasts.BMC Musculoskelet Disord. 2016 Nov 11;17(1):469. doi: 10.1186/s12891-016-1326-y. BMC Musculoskelet Disord. 2016. PMID: 27835939 Free PMC article.
-
Targeting GPCRs to treat cardiac fibrosis.Front Cardiovasc Med. 2022 Oct 6;9:1011176. doi: 10.3389/fcvm.2022.1011176. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36277752 Free PMC article. Review.
References
-
- Powell, D. W., Mifflin, R. C., Valentich, J. D., Crowe, S. E., Saada, J. I. & West, A. B. (1999) Am. J. Physiol. 277, C1-C9. - PubMed
-
- Weber, K. T. (1997) Semin. Nephrol. 17, 467-491. - PubMed
-
- Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C. & Brown, R. A. (2002) Nat. Rev. Mol. Cell. Biol. 3, 349-363. - PubMed
-
- Long, C. S. & Brown, R. D. (2002) J. Mol. Cell Cardiol. 34, 1273. - PubMed
-
- Kreis, T. E. & Birchmeier, W. (1980) Cell 22, 555-561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

