Mim1, a protein required for the assembly of the TOM complex of mitochondria
- PMID: 15608614
- PMCID: PMC1299228
- DOI: 10.1038/sj.embor.7400318
Mim1, a protein required for the assembly of the TOM complex of mitochondria
Abstract
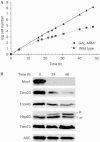
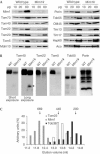
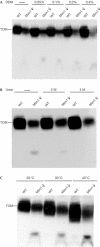
The translocase of the outer mitochondrial membrane (TOM complex) is the general entry site for newly synthesized proteins into mitochondria. This complex is essential for the formation and maintenance of mitochondria. Here, we report on the role of the integral outer membrane protein, Mim1 (mitochondrial import), in the biogenesis of mitochondria. Depletion of Mim1 abrogates assembly of the TOM complex and results in accumulation of Tom40, the principal constituent of the TOM complex, as a low-molecular-mass species. Like all mitochondrial beta-barrel proteins, the precursor of Tom40 is inserted into the outer membrane by the TOB complex. Mim1 is likely to be required for a step after this TOB-complex-mediated insertion. Mim1 is a constituent of neither the TOM complex nor the TOB complex; rather, it seems to be a subunit of another, as yet unidentified, complex. We conclude that Mim1 has a vital and specific function in the assembly of the TOM complex.
Figures


Similar articles
-
Biogenesis of the mitochondrial TOM complex: Mim1 promotes insertion and assembly of signal-anchored receptors.J Biol Chem. 2008 Jan 4;283(1):120-127. doi: 10.1074/jbc.M706997200. Epub 2007 Nov 1. J Biol Chem. 2008. PMID: 17974559
-
Assembly of the mitochondrial protein import channel: role of Tom5 in two-stage interaction of Tom40 with the SAM complex.Mol Biol Cell. 2010 Sep 15;21(18):3106-13. doi: 10.1091/mbc.E10-06-0518. Epub 2010 Jul 28. Mol Biol Cell. 2010. PMID: 20668160 Free PMC article.
-
Protein translocase of the outer mitochondrial membrane: role of import receptors in the structural organization of the TOM complex.J Mol Biol. 2002 Feb 22;316(3):657-66. doi: 10.1006/jmbi.2001.5365. J Mol Biol. 2002. PMID: 11866524
-
The enigmatic role of Mim1 in mitochondrial biogenesis.Eur J Cell Biol. 2010 Feb-Mar;89(2-3):212-5. doi: 10.1016/j.ejcb.2009.11.002. Epub 2009 Nov 26. Eur J Cell Biol. 2010. PMID: 19944477 Review.
-
The mitochondrial import machinery: preprotein-conducting channels with binding sites for presequences.Biochim Biophys Acta. 2002 Sep 2;1592(1):15-24. doi: 10.1016/s0167-4889(02)00260-4. Biochim Biophys Acta. 2002. PMID: 12191764 Review.
Cited by
-
Mcp3 is a novel mitochondrial outer membrane protein that follows a unique IMP-dependent biogenesis pathway.EMBO Rep. 2016 Jul;17(7):965-81. doi: 10.15252/embr.201541273. Epub 2016 May 24. EMBO Rep. 2016. PMID: 27226123 Free PMC article.
-
Outer membrane protein functions as integrator of protein import and DNA inheritance in mitochondria.Proc Natl Acad Sci U S A. 2016 Aug 2;113(31):E4467-75. doi: 10.1073/pnas.1605497113. Epub 2016 Jul 19. Proc Natl Acad Sci U S A. 2016. PMID: 27436903 Free PMC article.
-
The morphology proteins Mdm12/Mmm1 function in the major beta-barrel assembly pathway of mitochondria.EMBO J. 2007 May 2;26(9):2229-39. doi: 10.1038/sj.emboj.7601673. Epub 2007 Apr 5. EMBO J. 2007. PMID: 17410204 Free PMC article.
-
The Biogenesis of Mitochondrial Outer Membrane Proteins Show Variable Dependence on Import Factors.iScience. 2020 Jan 24;23(1):100779. doi: 10.1016/j.isci.2019.100779. Epub 2019 Dec 25. iScience. 2020. PMID: 31945731 Free PMC article.
-
Biogenesis of a Mitochondrial Outer Membrane Protein in Trypanosoma brucei: TARGETING SIGNAL AND DEPENDENCE ON A UNIQUE BIOGENESIS FACTOR.J Biol Chem. 2017 Feb 24;292(8):3400-3410. doi: 10.1074/jbc.M116.755983. Epub 2017 Jan 18. J Biol Chem. 2017. PMID: 28100781 Free PMC article.
References
-
- Daum G, Gasser SM, Schatz G (1982) Import of proteins into mitochondria. Energy-dependent, two-step processing of the intermembrane space enzyme cytochrome b2 by isolated yeast mitochondria. J Biol Chem 257: 13075–13080 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

