Growth inhibition by the tumor suppressor p33ING1 in immortalized and primary cells: involvement of two silencing domains and effect of Ras
- PMID: 15601862
- PMCID: PMC538761
- DOI: 10.1128/MCB.25.1.422-431.2005
Growth inhibition by the tumor suppressor p33ING1 in immortalized and primary cells: involvement of two silencing domains and effect of Ras
Abstract
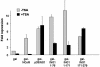
ING1 was identified as an inhibitor of growth and has been described as a tumor suppressor. Furthermore, the expression of ING1 is induced in senescent cells and antisense ING1 extends the proliferative life span of primary human fibroblasts. Cooperation of p33ING1 with p53 has been suggested to be an important function of ING1 in cell cycle control. Intriguingly, it has been shown that p33ING1 is associated with histone acetylation as well as with histone deacetylation function. Here we show that p33ING1 is a potent transcriptional silencer in various cell types. However, the silencing function is independent of the presence of p53. By use of deletion mutants two potent autonomous and transferable silencing domains were identified, but no evidence of an activation domain was found. The amino (N)-terminal silencing domain is sensitive to the histone deacetylase inhibitor trichostatin A (TSA) whereas the carboxy-terminal silencing function is resistant to TSA, suggesting that p33ING1 confers gene silencing through both HDAC-dependent and -independent mechanisms. Interestingly, the presence of oncogenic Ras, which is able to induce premature senescence, increases the p33ING1-mediated silencing function. Moreover, ING1-mediated silencing was reduced by coexpressing dominant-negative Ras or by treatment with the mitogen-activated protein kinase inhibitor PD98059 but not by treatment with SB203580, an inhibitor of the p38 pathway. In addition, we show that both silencing domains of ING1 are involved in cell cycle control, as measured by inhibition of colony formation of immortalized cells and by thymidine incorporation of primary human diploid fibroblasts (HDF). Interestingly, p33ING1 expression induces features of cellular senescence in HDFs.
Figures


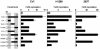
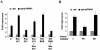
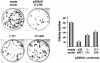


Similar articles
-
The candidate tumour suppressor p33ING1 cooperates with p53 in cell growth control.Nature. 1998 Jan 15;391(6664):295-8. doi: 10.1038/34675. Nature. 1998. PMID: 9440695
-
The tumor suppressor ING1 contributes to epigenetic control of cellular senescence.Aging Cell. 2011 Feb;10(1):158-71. doi: 10.1111/j.1474-9726.2010.00651.x. Epub 2010 Dec 13. Aging Cell. 2011. PMID: 21078114
-
The tumor suppressors p33ING1 and p33ING2 interact with alien in vivo and enhance alien-mediated gene silencing.J Proteome Res. 2007 Nov;6(11):4182-8. doi: 10.1021/pr070219d. Epub 2007 Oct 11. J Proteome Res. 2007. PMID: 17929852
-
Biological functions of the ING family tumor suppressors.Cell Mol Life Sci. 2004 Oct;61(19-20):2597-613. doi: 10.1007/s00018-004-4199-4. Cell Mol Life Sci. 2004. PMID: 15526165 Review.
-
Function of the ING family of PHD proteins in cancer.Int J Biochem Cell Biol. 2005 May;37(5):1054-65. doi: 10.1016/j.biocel.2004.09.008. Int J Biochem Cell Biol. 2005. PMID: 15743678 Review.
Cited by
-
Adenovirus-mediated expression of p33(ING1b) induces apoptosis and inhibits proliferation in gastric adenocarcinoma cells in vitro.Gastric Cancer. 2012 Oct;15(4):355-62. doi: 10.1007/s10120-011-0123-4. Epub 2012 Jan 12. Gastric Cancer. 2012. PMID: 22237655
-
Crystal structure of inhibitor of growth 4 (ING4) dimerization domain reveals functional organization of ING family of chromatin-binding proteins.J Biol Chem. 2012 Mar 30;287(14):10876-84. doi: 10.1074/jbc.M111.330001. Epub 2012 Feb 9. J Biol Chem. 2012. PMID: 22334692 Free PMC article.
-
Biological Functions of the ING Proteins.Cancers (Basel). 2019 Nov 19;11(11):1817. doi: 10.3390/cancers11111817. Cancers (Basel). 2019. PMID: 31752342 Free PMC article. Review.
-
ING Genes Work as Tumor Suppressor Genes in the Carcinogenesis of Head and Neck Squamous Cell Carcinoma.J Oncol. 2011;2011:963614. doi: 10.1155/2011/963614. Epub 2010 Oct 28. J Oncol. 2011. PMID: 21052543 Free PMC article.
-
Altered endocytosis in cellular senescence.Ageing Res Rev. 2021 Jul;68:101332. doi: 10.1016/j.arr.2021.101332. Epub 2021 Mar 19. Ageing Res Rev. 2021. PMID: 33753287 Free PMC article. Review.
References
-
- Baniahmad, A., C. Steiner, A. C. Kohne, and R. Renkawitz. 1990. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell 61:505-514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
