Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions
- PMID: 15601859
- PMCID: PMC538771
- DOI: 10.1128/MCB.25.1.389-402.2005
Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions
Abstract
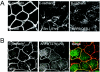
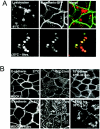
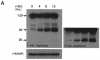
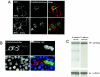
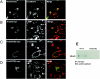
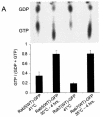
A hallmark characteristic of epithelial tumor progression as well as some processes of normal development is the loss of the epithelial phenotype and acquisition of a motile or mesenchymal phenotype. Such epithelial to mesenchymal transitions are accompanied by the loss of E-cadherin function by either transcriptional or posttranscriptional mechanisms. Here we demonstrate that, upon v-Src expression, a potent trigger of epithelial to mesenchymal transitions, E-cadherin is internalized and then shuttled to the lysosome instead of being recycled back to the lateral membrane. Thus, while E-cadherin internalization facilitates the dissolution of adherens junctions, its subsequent traffic to the lysosome serves as a means to ensure that cells do not reform their cell-cell contacts and remain motile. We also show that ubiquitin tagging of E-cadherin is essential for its sorting to the lysosome. The lysosomal targeting of E-cadherin is mediated by hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) and v-Src-induced activation of the Rab5 and Rab7 GTPases. Our studies reveal that the lysosomal targeting of E-cadherin is an important posttranscriptional mechanism to deplete cellular E-cadherin during Src-induced epithelial to mesenchymal transitions.
Figures






Similar articles
-
SRC-induced disintegration of adherens junctions of madin-darby canine kidney cells is dependent on endocytosis of cadherin and antagonized by Tiam-1.Lab Invest. 2003 Dec;83(12):1901-15. doi: 10.1097/01.lab.0000107009.75152.03. Lab Invest. 2003. PMID: 14691308
-
Cdc42 regulates E-cadherin ubiquitination and degradation through an epidermal growth factor receptor to Src-mediated pathway.J Biol Chem. 2008 Feb 22;283(8):5127-37. doi: 10.1074/jbc.M703300200. Epub 2007 Dec 5. J Biol Chem. 2008. PMID: 18057010
-
Activation of Rac by cadherin through the c-Src-Rap1-phosphatidylinositol 3-kinase-Vav2 pathway.Oncogene. 2006 Jan 5;25(1):8-19. doi: 10.1038/sj.onc.1209010. Oncogene. 2006. PMID: 16170364
-
The SRC-induced mesenchymal state in late-stage colon cancer cells.Cells Tissues Organs. 2005;179(1-2):73-80. doi: 10.1159/000084511. Cells Tissues Organs. 2005. PMID: 15942195 Review.
-
Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions.Cells Tissues Organs. 2007;185(1-3):73-84. doi: 10.1159/000101306. Cells Tissues Organs. 2007. PMID: 17587811 Review.
Cited by
-
Slit1b-Robo3 signaling and N-cadherin regulate apical process retraction in developing retinal ganglion cells.J Neurosci. 2012 Jan 4;32(1):223-8. doi: 10.1523/JNEUROSCI.2596-11.2012. J Neurosci. 2012. PMID: 22219284 Free PMC article.
-
The N-Myc down regulated Gene1 (NDRG1) Is a Rab4a effector involved in vesicular recycling of E-cadherin.PLoS One. 2007 Sep 5;2(9):e844. doi: 10.1371/journal.pone.0000844. PLoS One. 2007. PMID: 17786215 Free PMC article.
-
ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis.Semin Cell Dev Biol. 2011 Feb;22(1):39-47. doi: 10.1016/j.semcdb.2010.09.002. Epub 2010 Sep 15. Semin Cell Dev Biol. 2011. PMID: 20837153 Free PMC article. Review.
-
Functional ESCRT machinery is required for constitutive recycling of claudin-1 and maintenance of polarity in vertebrate epithelial cells.Mol Biol Cell. 2011 Sep;22(17):3192-205. doi: 10.1091/mbc.E11-04-0343. Epub 2011 Jul 14. Mol Biol Cell. 2011. PMID: 21757541 Free PMC article.
-
Peroxiredoxin stabilization of DE-cadherin promotes primordial germ cell adhesion.Dev Cell. 2011 Feb 15;20(2):233-43. doi: 10.1016/j.devcel.2010.12.007. Dev Cell. 2011. PMID: 21316590 Free PMC article.
References
-
- Behrens, J., L. Vakaet, R. Friis, E. Winterhager, F. Van Roy, M. M. Mareel, and W. Birchmeier. 1993. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120:757-766. - PMC - PubMed
-
- Birchmeier, W., J. Behrens, K. M. Weidner, J. Hulsken, and C. Birchmeier. 1996. Epithelial differentiation and the control of metastasis in carcinomas. Curr. Top. Microbiol. Immunol. 213:117-135. - PubMed
-
- Bolos, V., H. Peinado, M. A. Perez-Moreno, M. F. Fraga, M. Esteller, and A. Cano. 2003. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116:499-511. - PubMed
-
- Boyer, B., Y. Bourgeois, and M. F. Poupon. 2002. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene 21:2347-2356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
