Saccharomyces cerevisiae Aqr1 is an internal-membrane transporter involved in excretion of amino acids
- PMID: 15590823
- PMCID: PMC539038
- DOI: 10.1128/EC.3.6.1492-1503.2004
Saccharomyces cerevisiae Aqr1 is an internal-membrane transporter involved in excretion of amino acids
Abstract
Excretion of amino acids by yeast cells was reported long ago but has not been characterized in molecular terms. It is typically favored by overproduction of the amino acid and/or impairment of its uptake. Here we describe the construction of a yeast strain excreting threonine and homoserine. Using this excretor strain, we then applied a reverse-genetics approach and found that the transporter encoded by the YNL065w/AQR1 gene, a protein thought to mediate H(+) antiport, is involved in homoserine and threonine excretion. Furthermore, overexpression of AQR1 led to increased excretion of several amino acids (alanine, aspartate, and glutamate) known to be relatively abundant in the cytosol. Transcription of the AQR1 gene is induced severalfold by a number of amino acids and appears to be under the negative control of Gcn4. An Aqr1-green fluorescent protein fusion protein is located in multiple internal membrane structures and appears to cycle continuously between these compartments and the plasma membrane. The Aqr1 sequence is significantly similar to the vesicular amine transporters of secretory vesicles of neuronal cells. We propose that Aqr1 catalyzes transport of excess amino acids into vesicles, which then release them in the extracellular space by exocytosis.
Figures


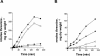
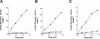


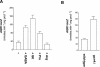

Similar articles
-
AQR1 gene (ORF YNL065w) encodes a plasma membrane transporter of the major facilitator superfamily that confers resistance to short-chain monocarboxylic acids and quinidine in Saccharomyces cerevisiae.Biochem Biophys Res Commun. 2002 Apr 5;292(3):741-8. doi: 10.1006/bbrc.2002.6703. Biochem Biophys Res Commun. 2002. PMID: 11922628
-
Overlapping Roles of Yeast Transporters Aqr1, Qdr2, and Qdr3 in Amino Acid Excretion and Cross-Feeding of Lactic Acid Bacteria.Front Microbiol. 2021 Nov 23;12:752742. doi: 10.3389/fmicb.2021.752742. eCollection 2021. Front Microbiol. 2021. PMID: 34887841 Free PMC article.
-
ATO3 encoding a putative outward ammonium transporter is an RTG-independent retrograde responsive gene regulated by GCN4 and the Ssy1-Ptr3-Ssy5 amino acid sensor system.J Biol Chem. 2003 Nov 14;278(46):45882-7. doi: 10.1074/jbc.M309301200. Epub 2003 Sep 9. J Biol Chem. 2003. PMID: 12966084
-
Transport of Amino Acids across the Vacuolar Membrane of Yeast: Its Mechanism and Physiological Role.Biol Pharm Bull. 2018;41(10):1496-1501. doi: 10.1248/bpb.b18-00165. Biol Pharm Bull. 2018. PMID: 30270317 Review.
-
Mitochondrial Carriers for Aspartate, Glutamate and Other Amino Acids: A Review.Int J Mol Sci. 2019 Sep 10;20(18):4456. doi: 10.3390/ijms20184456. Int J Mol Sci. 2019. PMID: 31510000 Free PMC article. Review.
Cited by
-
Ammonium toxicity and potassium limitation in yeast.PLoS Biol. 2006 Oct;4(11):e351. doi: 10.1371/journal.pbio.0040351. PLoS Biol. 2006. PMID: 17048990 Free PMC article.
-
Glutamine, arginine and the amino acid transporter Pt-CAT11 play important roles during senescence in poplar.Ann Bot. 2010 Jun;105(7):1159-69. doi: 10.1093/aob/mcq047. Epub 2010 Mar 17. Ann Bot. 2010. PMID: 20237111 Free PMC article.
-
Amino acid export in plants: a missing link in nitrogen cycling.Mol Plant. 2011 May;4(3):453-63. doi: 10.1093/mp/ssr003. Epub 2011 Feb 15. Mol Plant. 2011. PMID: 21324969 Free PMC article. Review.
-
Structural characterization of the Aspergillus niger citrate transporter CexA uncovers the role of key residues S75, R192 and Q196.Comput Struct Biotechnol J. 2023 Apr 26;21:2884-2898. doi: 10.1016/j.csbj.2023.04.025. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37216016 Free PMC article.
-
Dynamics of the Saccharomyces cerevisiae transcriptome during bread dough fermentation.Appl Environ Microbiol. 2013 Dec;79(23):7325-33. doi: 10.1128/AEM.02649-13. Epub 2013 Sep 20. Appl Environ Microbiol. 2013. PMID: 24056467 Free PMC article.
References
-
- Alarco, A. M., I. Balan, D. Talibi, N. Mainville, and M. Raymond. 1997. AP1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J. Biol. Chem. 272:19304-19313. - PubMed
-
- Albertsen, M., I. Bellahn, R. Kramer, and S. Waffenschmidt. 2003. Localization and function of the yeast multidrug transporter Tpo1p. J. Biol. Chem. 278:12820-12825. - PubMed
-
- André, B. 1995. An overview of membrane transport proteins in Saccharomyces cerevisiae. Yeast 11:1575-1611. - PubMed
-
- André, B., C. Hein, M. Grenson, and J. C. Jauniaux. 1993. Cloning and expression of the UGA4 gene coding for the inducible. Mol. Gen. Genet. 237:17-25. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

