SIGN-R1 contributes to protection against lethal pneumococcal infection in mice
- PMID: 15583012
- PMCID: PMC2211941
- DOI: 10.1084/jem.20040795
SIGN-R1 contributes to protection against lethal pneumococcal infection in mice
Abstract
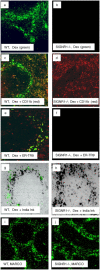
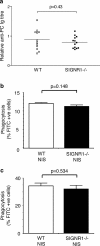
Rapid clearance of pathogens is essential for successful control of pyogenic bacterial infection. Previous experiments have shown that antibody to specific intracellular adhesion molecule-grabbing nonintegrin (SIGN)-R1 inhibits uptake of capsular polysaccharide by marginal zone macrophages, suggesting a role for SIGN-R1 in this process. We now demonstrate that mice lacking SIGN-R1 (a mouse homologue of human dendritic cell-SIGN receptor) are significantly more susceptible to Streptococcus pneumoniae infection and fail to clear S. pneumoniae from the circulation. Marginal zone and peritoneal macrophages show impaired bacterial recognition associated with an inability to bind T-independent type 2 antigens such as dextran. Our work represents the first evidence for a protective in vivo role for a SIGN family molecule.
Figures
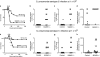
Similar articles
-
Specific ICAM-3 grabbing nonintegrin-related 1 (SIGNR1) expressed by marginal zone macrophages is essential for defense against pulmonary Streptococcus pneumoniae infection.Eur J Immunol. 2005 Oct;35(10):2962-9. doi: 10.1002/eji.200526216. Eur J Immunol. 2005. PMID: 16134084
-
Specific intracellular adhesion molecule-grabbing nonintegrin R1 is not involved in the murine antibody response to pneumococcal polysaccharides.Infect Immun. 2007 Dec;75(12):5748-52. doi: 10.1128/IAI.00574-07. Epub 2007 Sep 17. Infect Immun. 2007. PMID: 17875633 Free PMC article.
-
The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen.Proc Natl Acad Sci U S A. 2004 Jan 6;101(1):215-20. doi: 10.1073/pnas.0307124101. Epub 2003 Dec 23. Proc Natl Acad Sci U S A. 2004. PMID: 14694198 Free PMC article.
-
SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran.Int Immunol. 2003 Feb;15(2):177-86. doi: 10.1093/intimm/dxg019. Int Immunol. 2003. PMID: 12578847
-
DC-SIGN and mannosylated surface structures of Mycobacterium tuberculosis: a deceptive liaison.Eur J Cell Biol. 2010 Jan;89(1):95-101. doi: 10.1016/j.ejcb.2009.10.004. Epub 2009 Nov 4. Eur J Cell Biol. 2010. PMID: 19892432 Review.
Cited by
-
New insights into the cell biology of the marginal zone of the spleen.Int Rev Cytol. 2006;250:175-215. doi: 10.1016/S0074-7696(06)50005-1. Int Rev Cytol. 2006. PMID: 16861066 Free PMC article. Review.
-
Pneumococci: immunology of the innate host response.Respirology. 2010 Oct;15(7):1057-63. doi: 10.1111/j.1440-1843.2010.01814.x. Epub 2010 Jul 20. Respirology. 2010. PMID: 20646240 Free PMC article. Review.
-
Emergence of hypervirulent mutants resistant to early clearance during systemic serotype 1 pneumococcal infection in mice and humans.J Infect Dis. 2014 Jul 1;210(1):4-13. doi: 10.1093/infdis/jiu038. Epub 2014 Jan 16. J Infect Dis. 2014. PMID: 24443543 Free PMC article.
-
Association of SIGNR1 with TLR4-MD-2 enhances signal transduction by recognition of LPS in gram-negative bacteria.Int Immunol. 2005 Jul;17(7):827-36. doi: 10.1093/intimm/dxh264. Epub 2005 May 20. Int Immunol. 2005. PMID: 15908446 Free PMC article.
-
A sweet path toward tolerance in the gut.Nat Med. 2010 Oct;16(10):1076-7. doi: 10.1038/nm1010-1076. Nat Med. 2010. PMID: 20930744 No abstract available.
References
-
- Gordon, S. 2002. Pattern recognition receptors: doubling up for the innate immune response. Cell. 111:927–930. - PubMed
-
- Van Kooyk, Y., and T.B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697–709. - PubMed
-
- Geijtenbeek, T.B., R. Torensma, S.J. van Vliet, G.C. van Duijnhoven, G.J. Adema, Y. van Kooyk, and C.G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 100:575–585. - PubMed
-
- Geijtenbeek, T.B., D.S. Kwon, R. Torensma, S.J. van Vliet, G.C. van Duijnhoven, J. Middel, I.L. Cornelissen, H.S. Nottet, V.N. KewalRamani, D.R. Littman, C.G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 100:587–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

