The identities of sym-2, sym-3 and sym-4, three genes that are synthetically lethal with mec-8 in Caenorhabditis elegans
- PMID: 15579686
- PMCID: PMC1448807
- DOI: 10.1534/genetics.104.029827
The identities of sym-2, sym-3 and sym-4, three genes that are synthetically lethal with mec-8 in Caenorhabditis elegans
Abstract
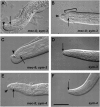
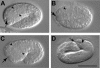
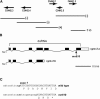
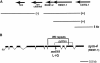
On the basis of synthetic lethality, five genes in Caenorhabditis elegans are known to be redundant with the mec-8 gene, which encodes a protein that contains two copies of an RNA recognition motif (RRM) and affects alternative RNA splicing. The molecular identities of two of the redundant genes, sym-1 and sym-5, were previously reported. The remaining three genes have now been cloned, and their synthetically lethal phenotypes with mec-8 are described in more detail. Animals homozygous for mec-8 and sym-2 loss-of-function mutations die during late embryogenesis. The SYM-2 predicted protein contains three RRMs; we propose that SYM-2 and MEC-8 can substitute for each other in promoting the maturation of the transcripts of a vital gene. Animals homozygous for mutations in mec-8 and in either sym-3 or sym-4 have the same striking defect: they arrest development just prior to or just after hatching with a pharynx that appears fully formed but is not properly attached to the body cuticle. sym-3 encodes a protein of unknown function with orthologs in Drosophila and mammals. sym-4 encodes a WD-repeat protein and may also have orthologs in Drosophila and mammals. We propose that SYM-3 and SYM-4 contribute to a common developmental pathway that is redundant with a MEC-8-dependent pathway.
Figures





Similar articles
-
Functional overlap between the mec-8 gene and five sym genes in Caenorhabditis elegans.Genetics. 1999 Sep;153(1):117-34. doi: 10.1093/genetics/153.1.117. Genetics. 1999. PMID: 10471705 Free PMC article.
-
The mec-8 gene of C. elegans encodes a protein with two RNA recognition motifs and regulates alternative splicing of unc-52 transcripts.Development. 1996 May;122(5):1601-10. doi: 10.1242/dev.122.5.1601. Development. 1996. PMID: 8625846
-
Conserved binding of GCAC motifs by MEC-8, couch potato, and the RBPMS protein family.RNA. 2017 Mar;23(3):308-316. doi: 10.1261/rna.059733.116. Epub 2016 Dec 21. RNA. 2017. PMID: 28003515 Free PMC article.
-
MEC-8 regulates alternative splicing of unc-52 transcripts in C. elegans hypodermal cells.Development. 2002 Nov;129(21):4999-5008. doi: 10.1242/dev.129.21.4999. Development. 2002. PMID: 12397108
-
C. elegans sym-1 is a downstream target of the hunchback-like-1 developmental timing transcription factor.Cell Cycle. 2009 Dec 15;8(24):4147-54. doi: 10.4161/cc.8.24.10292. Epub 2009 Dec 9. Cell Cycle. 2009. PMID: 19923914 Free PMC article.
Cited by
-
The Caenorhabditis elegans epidermis as a model skin. II: differentiation and physiological roles.Wiley Interdiscip Rev Dev Biol. 2012 Nov-Dec;1(6):879-902. doi: 10.1002/wdev.77. Epub 2012 Jun 19. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23539358 Free PMC article. Review.
-
Switch-like regulation of tissue-specific alternative pre-mRNA processing patterns revealed by customized fluorescence reporters.Worm. 2013 Jul 1;2(3):e23834. doi: 10.4161/worm.23834. Epub 2013 Oct 25. Worm. 2013. PMID: 24778931 Free PMC article.
-
Complex changes in alternative pre-mRNA splicing play a central role in the epithelial-to-mesenchymal transition (EMT).Semin Cancer Biol. 2012 Oct;22(5-6):417-27. doi: 10.1016/j.semcancer.2012.04.003. Epub 2012 Apr 23. Semin Cancer Biol. 2012. PMID: 22548723 Free PMC article. Review.
-
Conserved RNA-binding proteins required for dendrite morphogenesis in Caenorhabditis elegans sensory neurons.G3 (Bethesda). 2015 Feb 10;5(4):639-53. doi: 10.1534/g3.115.017327. G3 (Bethesda). 2015. PMID: 25673135 Free PMC article.
-
Touch sensitivity in Caenorhabditis elegans.Pflugers Arch. 2007 Aug;454(5):691-702. doi: 10.1007/s00424-006-0187-x. Epub 2007 Feb 7. Pflugers Arch. 2007. PMID: 17285303 Review.
References
-
- Aroian, R. V., M. Koga, J. E. Mendel, Y. Ohshima and P. W. Sternberg, 1990. The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature 348: 693–699. - PubMed
-
- Blumenthal, T., and K. Steward, 1997 RNA processing and gene structure, pp. 117–145 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Plainview, NY. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

