Transplanted hematopoietic stem cells demonstrate impaired sarcoglycan expression after engraftment into cardiac and skeletal muscle
- PMID: 15578090
- PMCID: PMC529287
- DOI: 10.1172/JCI23071
Transplanted hematopoietic stem cells demonstrate impaired sarcoglycan expression after engraftment into cardiac and skeletal muscle
Abstract
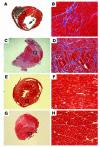
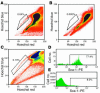
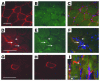
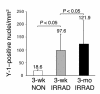
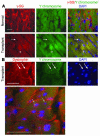
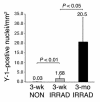
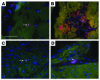
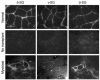
Pluripotent bone marrow-derived side population (BM-SP) stem cells have been shown to repopulate the hematopoietic system and to contribute to skeletal and cardiac muscle regeneration after transplantation. We tested BM-SP cells for their ability to regenerate heart and skeletal muscle using a model of cardiomyopathy and muscular dystrophy that lacks delta-sarcoglycan. The absence of delta-sarcoglycan produces microinfarcts in heart and skeletal muscle that should recruit regenerative stem cells. Additionally, sarcoglycan expression after transplantation should mark successful stem cell maturation into cardiac and skeletal muscle lineages. BM-SP cells from normal male mice were transplanted into female delta-sarcoglycan-null mice. We detected engraftment of donor-derived stem cells into skeletal muscle, with the majority of donor-derived cells incorporated within myofibers. In the heart, donor-derived nuclei were detected inside cardiomyocytes. Skeletal muscle myofibers containing donor-derived nuclei generally failed to express sarcoglycan, with only 2 sarcoglycan-positive fibers detected in the quadriceps muscle from all 14 mice analyzed. Moreover, all cardiomyocytes with donor-derived nuclei were sarcoglycan-negative. The absence of sarcoglycan expression in cardiomyocytes and skeletal myofibers after transplantation indicates impaired differentiation and/or maturation of bone marrow-derived stem cells. The inability of BM-SP cells to express this protein severely limits their utility for cardiac and skeletal muscle regeneration.
Figures
Comment in
-
Fusion of bone marrow-derived stem cells with striated muscle may not be sufficient to activate muscle genes.J Clin Invest. 2004 Dec;114(11):1540-3. doi: 10.1172/JCI23733. J Clin Invest. 2004. PMID: 15578085 Free PMC article.
Similar articles
-
Fusion of bone marrow-derived stem cells with striated muscle may not be sufficient to activate muscle genes.J Clin Invest. 2004 Dec;114(11):1540-3. doi: 10.1172/JCI23733. J Clin Invest. 2004. PMID: 15578085 Free PMC article.
-
Determinants of skeletal muscle contributions from circulating cells, bone marrow cells, and hematopoietic stem cells.Stem Cells. 2004;22(7):1292-304. doi: 10.1634/stemcells.2004-0090. Stem Cells. 2004. PMID: 15579647
-
Skeletal myosphere-derived progenitor cell transplantation promotes neovascularization in delta-sarcoglycan knockdown cardiomyopathy.Biochem Biophys Res Commun. 2007 Jan 19;352(3):668-74. doi: 10.1016/j.bbrc.2006.11.097. Epub 2006 Nov 27. Biochem Biophys Res Commun. 2007. PMID: 17150187
-
Endogenous cardiac stem cells.Prog Cardiovasc Dis. 2007 Jul-Aug;50(1):31-48. doi: 10.1016/j.pcad.2007.03.005. Prog Cardiovasc Dis. 2007. PMID: 17631436 Review.
-
Regeneration gaps: observations on stem cells and cardiac repair.J Am Coll Cardiol. 2006 May 2;47(9):1777-85. doi: 10.1016/j.jacc.2006.02.002. Epub 2006 Apr 17. J Am Coll Cardiol. 2006. PMID: 16682301 Review.
Cited by
-
Identification and Characterization of the Dermal Panniculus Carnosus Muscle Stem Cells.Stem Cell Reports. 2016 Sep 13;7(3):411-424. doi: 10.1016/j.stemcr.2016.08.002. Epub 2016 Sep 1. Stem Cell Reports. 2016. PMID: 27594590 Free PMC article.
-
G-CSF/SCF reduces inducible arrhythmias in the infarcted heart potentially via increased connexin43 expression and arteriogenesis.J Exp Med. 2006 Jan 23;203(1):87-97. doi: 10.1084/jem.20051151. Epub 2006 Jan 9. J Exp Med. 2006. PMID: 16401694 Free PMC article.
-
Limb-girdle muscular dystrophies: where next after six decades from the first proposal (Review).Mol Med Rep. 2014 May;9(5):1515-32. doi: 10.3892/mmr.2014.2048. Epub 2014 Mar 13. Mol Med Rep. 2014. PMID: 24626787 Free PMC article. Review.
-
Reflections on lineage potential of skeletal muscle satellite cells: do they sometimes go MAD?Crit Rev Eukaryot Gene Expr. 2007;17(1):13-29. doi: 10.1615/critreveukargeneexpr.v17.i1.20. Crit Rev Eukaryot Gene Expr. 2007. PMID: 17341181 Free PMC article. Review.
-
Microcarrier-based expansion of adult murine side population stem cells.PLoS One. 2013;8(1):e55187. doi: 10.1371/journal.pone.0055187. Epub 2013 Jan 28. PLoS One. 2013. PMID: 23383102 Free PMC article.
References
-
- Frisen J. Stem cell plasticity? Neuron. 2002;35:415–418. - PubMed
-
- Morrison SJ. Stem cell potential: can anything make anything? Curr. Biol. 2001;11:R7–R9. - PubMed
-
- Corti S, et al. A subpopulation of murine bone marrow cells fully differentiates along the myogenic pathway and participates in muscle repair in the mdx dystrophic mouse. Exp. Cell Res. 2002;277:74–85. - PubMed
-
- LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. - PubMed
-
- Camargo FD, Green R, Capetenaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat. Med. 2003;9:1520–1527. - PubMed

