Fusion of bone marrow-derived stem cells with striated muscle may not be sufficient to activate muscle genes
- PMID: 15578085
- PMCID: PMC529291
- DOI: 10.1172/JCI23733
Fusion of bone marrow-derived stem cells with striated muscle may not be sufficient to activate muscle genes
Abstract
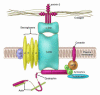
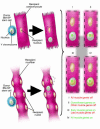
Several studies have demonstrated the existence of pluripotent bone marrow-derived stem cells capable of homing to injured cardiac and skeletal muscle; however, there has been little evidence demonstrating the induction of tissue-specific endogenous genes in donor stem cells following engraftment. A new study in this issue reports an intriguing finding that raises additional concerns relating to stem cell plasticity and stem cell therapy in an already heated and controversial field. The study demonstrates that wild-type bone marrow-derived side population stem cells are indeed readily incorporated into both skeletal and cardiac muscle when transplanted into mice that lack delta-sarcoglycan -- a model of cardiomyopathy and muscular dystrophy. However, these cells fail to express sarcoglycan and thus to repair the tissue, which suggests that this stem cell population has limited potential for cardiac and skeletal muscle regeneration.
Figures
Comment on
-
Transplanted hematopoietic stem cells demonstrate impaired sarcoglycan expression after engraftment into cardiac and skeletal muscle.J Clin Invest. 2004 Dec;114(11):1577-85. doi: 10.1172/JCI23071. J Clin Invest. 2004. PMID: 15578090 Free PMC article.
Similar articles
-
Transplanted hematopoietic stem cells demonstrate impaired sarcoglycan expression after engraftment into cardiac and skeletal muscle.J Clin Invest. 2004 Dec;114(11):1577-85. doi: 10.1172/JCI23071. J Clin Invest. 2004. PMID: 15578090 Free PMC article.
-
Determinants of skeletal muscle contributions from circulating cells, bone marrow cells, and hematopoietic stem cells.Stem Cells. 2004;22(7):1292-304. doi: 10.1634/stemcells.2004-0090. Stem Cells. 2004. PMID: 15579647
-
Contribution of hematopoietic stem cells to skeletal muscle.Nat Med. 2003 Dec;9(12):1528-32. doi: 10.1038/nm959. Epub 2003 Nov 16. Nat Med. 2003. PMID: 14625543
-
A new look at the origin, function, and "stem-cell" status of muscle satellite cells.Dev Biol. 2000 Feb 15;218(2):115-24. doi: 10.1006/dbio.1999.9565. Dev Biol. 2000. PMID: 10656756 Review.
-
Bone marrow-derived stem cells and "plasticity".Ann Hematol. 2003 Oct;82(10):599-604. doi: 10.1007/s00277-003-0713-2. Epub 2003 Jul 24. Ann Hematol. 2003. PMID: 12898189 Review.
Cited by
-
Mesenchymal stem cells: emerging therapy for Duchenne muscular dystrophy.PM R. 2009 Jun;1(6):547-59. doi: 10.1016/j.pmrj.2009.02.013. PM R. 2009. PMID: 19627945 Free PMC article. Review.
-
Growth of bone marrow and skeletal muscle side population stem cells in suspension culture.Methods Mol Biol. 2014;1210:51-61. doi: 10.1007/978-1-4939-1435-7_5. Methods Mol Biol. 2014. PMID: 25173160 Free PMC article.
-
Identification and Characterization of the Dermal Panniculus Carnosus Muscle Stem Cells.Stem Cell Reports. 2016 Sep 13;7(3):411-424. doi: 10.1016/j.stemcr.2016.08.002. Epub 2016 Sep 1. Stem Cell Reports. 2016. PMID: 27594590 Free PMC article.
-
Accumulation of Dystrophin-Positive Muscle Fibers and Improvement of Neuromuscular Junctions in mdx Mouse Muscles after Bone Marrow Transplantation under Different Conditions.Int J Mol Sci. 2023 May 17;24(10):8892. doi: 10.3390/ijms24108892. Int J Mol Sci. 2023. PMID: 37240237 Free PMC article.
References
-
- Gussoni E, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. - PubMed
-
- Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. - PubMed
-
- Coral-Vazquez R, et al. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999;98:465–474. - PubMed
-
- Seale P, Rudnicki MA. A new look at the origin, function, and “stemcell” status of muscle satellite cells. Dev. Biol. 2000;218:115–124. - PubMed

