Molecular constraints to interspecies transmission of viral pathogens
- PMID: 15577935
- PMCID: PMC7095872
- DOI: 10.1038/nm1151
Molecular constraints to interspecies transmission of viral pathogens
Abstract
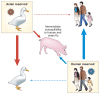
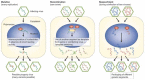
The successful replication of a viral pathogen in a host is a complex process involving many interactions. These interactions develop from the coevolution of pathogen and host and often lead to a species specificity of the virus that can make interspecies transmissions difficult. Nevertheless, viruses do sporadically cross species barriers into other host populations, including humans. In zoonotic infections, many of these interspecies transfer events are dead end, where transmission is confined only to the animal-to-human route but sometimes viruses adapt to enable spread from human to human. A pathogen must overcome many hurdles to replicate successfully in a foreign host. The viral pathogen must enter the host cell, replicate with the assistance of host factors, evade inhibitory host products, exit the first cell and move on to the next, and possibly leave the initial host and transmit to another. Each of these stages may require adaptive changes in the pathogen. Although the factors that influence each stage of the replication and transmission of most agents have not been resolved, the genomics of both hosts and pathogens are now at hand and we have begun to understand some of the molecular changes that enable some viruses to adapt to a new host.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
[Mechanisms of viral emergence and interspecies transmission: the exemple of simian foamy viruses in Central Africa].Bull Acad Natl Med. 2013 Dec;197(9):1655-67; discussion 1667-8. doi: 10.1016/S0001-4079(19)31387-1. Bull Acad Natl Med. 2013. PMID: 26137812 Free PMC article. Review. French.
-
Bats as Viral Reservoirs.Annu Rev Virol. 2016 Sep 29;3(1):77-99. doi: 10.1146/annurev-virology-110615-042203. Epub 2016 Aug 22. Annu Rev Virol. 2016. PMID: 27578437 Review.
-
Accelerated viral dynamics in bat cell lines, with implications for zoonotic emergence.Elife. 2020 Feb 3;9:e48401. doi: 10.7554/eLife.48401. Elife. 2020. PMID: 32011232 Free PMC article.
-
Animal virus discovery: improving animal health, understanding zoonoses, and opportunities for vaccine development.Curr Opin Virol. 2012 Jun;2(3):344-52. doi: 10.1016/j.coviro.2012.02.012. Epub 2012 Mar 15. Curr Opin Virol. 2012. PMID: 22463981 Free PMC article. Review.
-
Introduction: conceptualizing and partitioning the emergence process of zoonotic viruses from wildlife to humans.Curr Top Microbiol Immunol. 2007;315:1-31. doi: 10.1007/978-3-540-70962-6_1. Curr Top Microbiol Immunol. 2007. PMID: 17848058 Free PMC article. Review.
Cited by
-
Avian influenza viruses infect primary human bronchial epithelial cells unconstrained by sialic acid α2,3 residues.PLoS One. 2011;6(6):e21183. doi: 10.1371/journal.pone.0021183. Epub 2011 Jun 23. PLoS One. 2011. PMID: 21731666 Free PMC article.
-
Expected Effect of Deleterious Mutations on Within-Host Adaptation of Pathogens.J Virol. 2015 Sep;89(18):9242-51. doi: 10.1128/JVI.00832-15. Epub 2015 Jun 24. J Virol. 2015. PMID: 26109724 Free PMC article.
-
Emerging and re-emerging infections at the turn of the millennium.Haemophilia. 2010 Jan;16 Suppl 1(Suppl 1):7-12. doi: 10.1111/j.1365-2516.2009.02174.x. Haemophilia. 2010. PMID: 20059563 Free PMC article. Review.
-
Inefficient transmission of H5N1 influenza viruses in a ferret contact model.J Virol. 2007 Jul;81(13):6890-8. doi: 10.1128/JVI.00170-07. Epub 2007 Apr 25. J Virol. 2007. PMID: 17459930 Free PMC article.
-
Gripe y viriasis respiratorias.Medicine (Madr). 2010 Jun;10(58):3958-3967. doi: 10.1016/S0304-5412(10)70146-3. Epub 2010 Jul 7. Medicine (Madr). 2010. PMID: 32308251 Free PMC article. Spanish. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

