Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin
- PMID: 15572663
- PMCID: PMC533963
- DOI: 10.1128/MCB.24.24.10558-10572.2004
Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin
Abstract
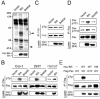
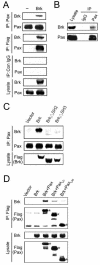
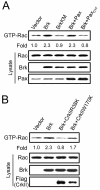
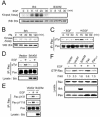
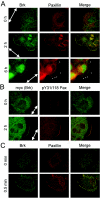
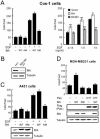
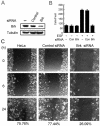
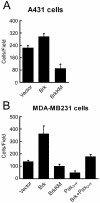
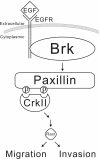
Brk (for breast tumor kinase) is a nonreceptor tyrosine kinase containing SH3, SH2, and tyrosine kinase catalytic domains. Brk was originally identified from a human metastatic breast tumor, and its overexpression is frequently observed in breast cancer and several other cancer types. However, the molecular mechanism by which this kinase participates in tumorigenesis remains poorly characterized. In the present study, we not only identified paxillin as the binding partner and substrate of Brk but also discovered a novel signaling pathway by which Brk mediates epidermal growth factor (EGF)-induced paxillin phosphorylation. We show that EGF stimulation activates the catalytic activity of Brk, which in turn phosphorylates paxillin at Y31 and Y118. These phosphorylation events promote the activation of small GTPase Rac1 via the function of CrkII. Through this pathway, Brk is capable of promoting cell motility and invasion and functions as a mediator of EGF-induced migration and invasion. In accordance with these functional roles, Brk translocates to membrane ruffles, where it colocalizes with paxillin during cell migration. Together, our findings identify novel signaling and biological roles of Brk and indicate the first potential link between Brk and metastatic malignancy.
Figures


Similar articles
-
Tyrosine phosphorylation of the CrkII adaptor protein modulates cell migration.J Cell Sci. 2003 Aug 1;116(Pt 15):3145-55. doi: 10.1242/jcs.00632. Epub 2003 Jun 10. J Cell Sci. 2003. PMID: 12799422
-
Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells.Cancer Res. 2007 May 1;67(9):4199-209. doi: 10.1158/0008-5472.CAN-06-3409. Cancer Res. 2007. PMID: 17483331
-
Coupling of RAFTK/Pyk2 kinase with c-Abl and their role in the migration of breast cancer cells.Int J Oncol. 2004 Jan;24(1):153-9. Int J Oncol. 2004. PMID: 14654952
-
Understanding the cellular roles of Fyn-related kinase (FRK): implications in cancer biology.Cancer Metastasis Rev. 2016 Jun;35(2):179-99. doi: 10.1007/s10555-016-9623-3. Cancer Metastasis Rev. 2016. PMID: 27067725 Review.
-
Tracing the footprints of the breast cancer oncogene BRK - Past till present.Biochim Biophys Acta. 2015 Aug;1856(1):39-54. doi: 10.1016/j.bbcan.2015.05.001. Epub 2015 May 18. Biochim Biophys Acta. 2015. PMID: 25999240 Review.
Cited by
-
Low expression of PTK6/Brk predicts poor prognosis in patients with laryngeal squamous cell carcinoma.J Transl Med. 2013 Mar 7;11:59. doi: 10.1186/1479-5876-11-59. J Transl Med. 2013. PMID: 23497344 Free PMC article.
-
PTK6 regulates IGF-1-induced anchorage-independent survival.PLoS One. 2010 Jul 23;5(7):e11729. doi: 10.1371/journal.pone.0011729. PLoS One. 2010. PMID: 20668531 Free PMC article.
-
Cytoplasmic retention of protein tyrosine kinase 6 promotes growth of prostate tumor cells.Cell Cycle. 2010 Oct 15;9(20):4190-9. doi: 10.4161/cc.9.20.13518. Epub 2010 Oct 30. Cell Cycle. 2010. PMID: 20953141 Free PMC article.
-
Context-specific protein tyrosine kinase 6 (PTK6) signalling in prostate cancer.Eur J Clin Invest. 2013 Apr;43(4):397-404. doi: 10.1111/eci.12050. Epub 2013 Feb 10. Eur J Clin Invest. 2013. PMID: 23398121 Free PMC article. Review.
-
Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells.J Biol Chem. 2010 Sep 10;285(37):28787-95. doi: 10.1074/jbc.M110.134064. Epub 2010 Jul 13. J Biol Chem. 2010. PMID: 20628053 Free PMC article.
References
-
- Avraham, H., S. Y. Park, K. Schinkmann, and S. Avraham. 2000. RAFTK/Pyk2-mediated cellular signalling. Cell Signal 12:123-133. - PubMed
-
- Bagrodia, S., S. J. Taylor, K. A. Jordon, L. Van Aelst, and R. A. Cerione. 1998. A novel regulator of p21-activated kinases. J. Biol. Chem. 273:23633-23636. - PubMed
-
- Barker, K. T., L. E. Jackson, and M. R. Crompton. 1997. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene 15:799-805. - PubMed
-
- Bar-Sagi, D., and A. Hall. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227-238. - PubMed
-
- Bellis, S. L., J. T. Miller, and C. E. Turner. 1995. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J. Biol. Chem. 270:17437-17441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
