A green fluorescent protein fusion to actin-binding domain 2 of Arabidopsis fimbrin highlights new features of a dynamic actin cytoskeleton in live plant cells
- PMID: 15557099
- PMCID: PMC535829
- DOI: 10.1104/pp.104.049411
A green fluorescent protein fusion to actin-binding domain 2 of Arabidopsis fimbrin highlights new features of a dynamic actin cytoskeleton in live plant cells
Abstract
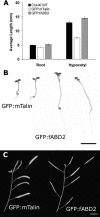
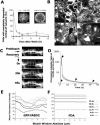
The actin cytoskeleton coordinates numerous cellular processes required for plant development. The functions of this network are intricately linked to its dynamic arrangement, and thus progress in understanding how actin orchestrates cellular processes relies on critical evaluation of actin organization and turnover. To investigate the dynamic nature of the actin cytoskeleton, we used a fusion protein between green fluorescent protein (GFP) and the second actin-binding domain (fABD2) of Arabidopsis (Arabidopsis thaliana) fimbrin, AtFIM1. The GFP-fABD2 fusion protein labeled highly dynamic and dense actin networks in diverse species and cell types, revealing structural detail not seen with alternative labeling methods, such as the commonly used mouse talin GFP fusion (GFP-mTalin). Further, we show that expression of the GFP-fABD2 fusion protein in Arabidopsis, unlike GFP-mTalin, has no detectable adverse effects on plant morphology or development. Time-lapse confocal microscopy and fluorescence recovery after photobleaching analyses of the actin cytoskeleton labeled with GFP-fABD2 revealed that lateral-filament migration and sliding of individual actin filaments or bundles are processes that contribute to the dynamic and continually reorganizing nature of the actin scaffold. These new observations of the dynamic actin cytoskeleton in plant cells using GFP-fABD2 reveal the value of this probe for future investigations of how actin filaments coordinate cellular processes required for plant development.
Figures



Similar articles
-
Green fluorescent protein fusions to Arabidopsis fimbrin 1 for spatio-temporal imaging of F-actin dynamics in roots.Cell Motil Cytoskeleton. 2004 Oct;59(2):79-93. doi: 10.1002/cm.20024. Cell Motil Cytoskeleton. 2004. PMID: 15362112
-
Green fluorescent protein-mTalin causes defects in actin organization and cell expansion in Arabidopsis and inhibits actin depolymerizing factor's actin depolymerizing activity in vitro.Plant Physiol. 2004 Dec;136(4):3990-8. doi: 10.1104/pp.104.050799. Epub 2004 Nov 24. Plant Physiol. 2004. PMID: 15563618 Free PMC article.
-
High expression of Lifeact in Arabidopsis thaliana reduces dynamic reorganization of actin filaments but does not affect plant development.Cytoskeleton (Hoboken). 2011 Oct;68(10):578-87. doi: 10.1002/cm.20534. Epub 2011 Oct 4. Cytoskeleton (Hoboken). 2011. PMID: 21948789
-
Actin dynamics in the cortical array of plant cells.Curr Opin Plant Biol. 2013 Dec;16(6):678-87. doi: 10.1016/j.pbi.2013.10.012. Epub 2013 Nov 15. Curr Opin Plant Biol. 2013. PMID: 24246228 Review.
-
Actin-myosin XI: an intracellular control network in plants.Biochem Biophys Res Commun. 2018 Nov 25;506(2):403-408. doi: 10.1016/j.bbrc.2017.12.169. Epub 2018 Jan 5. Biochem Biophys Res Commun. 2018. PMID: 29307817 Review.
Cited by
-
The plant actin cytoskeleton responds to signals from microbe-associated molecular patterns.PLoS Pathog. 2013;9(4):e1003290. doi: 10.1371/journal.ppat.1003290. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23593000 Free PMC article.
-
mEosFP-based green-to-red photoconvertible subcellular probes for plants.Plant Physiol. 2010 Dec;154(4):1573-87. doi: 10.1104/pp.110.165431. Epub 2010 Oct 12. Plant Physiol. 2010. PMID: 20940350 Free PMC article.
-
Arabidopsis LIM proteins: a family of actin bundlers with distinct expression patterns and modes of regulation.Plant Cell. 2010 Sep;22(9):3034-52. doi: 10.1105/tpc.110.075960. Epub 2010 Sep 3. Plant Cell. 2010. PMID: 20817848 Free PMC article.
-
Inhibition of tobacco mosaic virus movement by expression of an actin-binding protein.Plant Physiol. 2009 Apr;149(4):1810-23. doi: 10.1104/pp.108.133827. Epub 2009 Feb 13. Plant Physiol. 2009. PMID: 19218363 Free PMC article.
-
Evolutionarily unique mechanistic framework of clathrin-mediated endocytosis in plants.Elife. 2020 Jan 23;9:e52067. doi: 10.7554/eLife.52067. Elife. 2020. PMID: 31971511 Free PMC article.
References
-
- Baluska F, Jasik J, Edelmann HG, Salajova T, Volkmann D (2001) Latrunculin B-induced plant dwarfism: plant cell elongation is F-actin-dependent. Dev Biol 231: 113–124 - PubMed
-
- Baluska F, Wojtaszek P, Volkmann D, Barlow P (2003) The architecture of polarized cell growth: the unique status of elongating plant cells. Bioessays 25: 569–576 - PubMed
-
- Blanchoin L, Pollard TD, Mullins RD (2000) Interactions of ADF/cofilin, Arp2/3 complex, capping protein and profilin in remodeling of branched actin filament networks. Curr Biol 10: 1273–1282 - PubMed
-
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 - PubMed
-
- El-Assal SE, Le J, Basu D, Mallery EL, Szymanski DB (2004) DISTORTED encodes an ARPC2 subunit of the putative Arabidopsis ARP2/3 complex. Plant J 38: 526–538 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

