Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells
- PMID: 15549136
- PMCID: PMC533055
- DOI: 10.1038/sj.emboj.7600471
Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells
Abstract
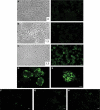
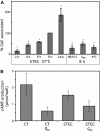
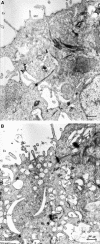
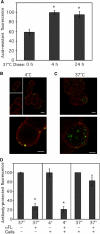
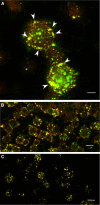
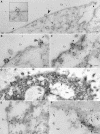
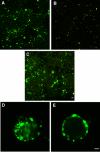
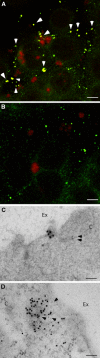
Enterotoxigenic Escherichia coli (ETEC) is a prevalent cause of traveler's diarrhea and infant mortality in third-world countries. Heat-labile enterotoxin (LT) is secreted from ETEC via vesicles composed of outer membrane and periplasm. We investigated the role of ETEC vesicles in pathogenesis by analyzing vesicle association and entry into eukaryotic cells. Fluorescently labeled vesicles from LT-producing and LT-nonproducing strains were compared in their ability to bind adrenal and intestinal epithelial cells. ETEC-derived vesicles, but not control nonpathogen-derived vesicles, associated with cells in a time-, temperature-, and receptor-dependent manner. Vesicles were visualized on the cell surface at 4 degrees C and detected intracellularly at 37 degrees C. ETEC vesicle endocytosis depended on cholesterol-rich lipid rafts. Entering vesicles partially colocalized with caveolin, and the internalized vesicles accumulated in a nonacidified compartment. We conclude that ETEC vesicles serve as specifically targeted transport vehicles that mediate entry of active enterotoxin and other bacterial envelope components into host cells. These data demonstrate a role in virulence for ETEC vesicles.
Figures

Similar articles
-
Context-dependent activation kinetics elicited by soluble versus outer membrane vesicle-associated heat-labile enterotoxin.Infect Immun. 2011 Sep;79(9):3760-9. doi: 10.1128/IAI.05336-11. Epub 2011 Jun 27. Infect Immun. 2011. PMID: 21708992 Free PMC article.
-
Porcine Enterotoxigenic Escherichia coli Strains Differ in Their Capacity To Secrete Enterotoxins through Varying YghG Levels.Appl Environ Microbiol. 2020 Nov 24;86(24):e00523-20. doi: 10.1128/AEM.00523-20. Print 2020 Nov 24. Appl Environ Microbiol. 2020. PMID: 32561576 Free PMC article.
-
Biochemical characterization of the enterotoxigenic Escherichia coli LeoA protein.Microbiology (Reading). 2007 Nov;153(Pt 11):3776-3784. doi: 10.1099/mic.0.2007/009084-0. Microbiology (Reading). 2007. PMID: 17975086
-
Secretion and Delivery of Intestinal Pathogenic Escherichia coli Virulence Factors via Outer Membrane Vesicles.Front Cell Infect Microbiol. 2020 Mar 6;10:91. doi: 10.3389/fcimb.2020.00091. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32211344 Free PMC article. Review.
-
Adhesion of enterotoxigenic Escherichia coli in humans and animals.Ciba Found Symp. 1981;80:142-60. doi: 10.1002/9780470720639.ch10. Ciba Found Symp. 1981. PMID: 6114818 Review.
Cited by
-
The potential role of gut microbiota outer membrane vesicles in colorectal cancer.Front Microbiol. 2023 Nov 7;14:1270158. doi: 10.3389/fmicb.2023.1270158. eCollection 2023. Front Microbiol. 2023. PMID: 38029123 Free PMC article. Review.
-
Biochemical and functional characterization of Helicobacter pylori vesicles.Mol Microbiol. 2010 Sep;77(6):1539-55. doi: 10.1111/j.1365-2958.2010.07307.x. Epub 2010 Aug 5. Mol Microbiol. 2010. PMID: 20659286 Free PMC article.
-
Extracellular vesicles participate in the pathogenesis of sepsis.Front Cell Infect Microbiol. 2022 Dec 12;12:1018692. doi: 10.3389/fcimb.2022.1018692. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36579343 Free PMC article. Review.
-
Role of Shiga Toxins in Cytotoxicity and Immunomodulatory Effects of Escherichia coli O157:H7 during Host-Bacterial Interactions in vitro.Toxins (Basel). 2020 Jan 14;12(1):48. doi: 10.3390/toxins12010048. Toxins (Basel). 2020. PMID: 31947665 Free PMC article.
-
Residues of heat-labile enterotoxin involved in bacterial cell surface binding.J Bacteriol. 2009 May;191(9):2917-25. doi: 10.1128/JB.01622-08. Epub 2009 Mar 6. J Bacteriol. 2009. PMID: 19270095 Free PMC article.
References
-
- Anderson RG, Kamen BA, Rothberg KG, Lacey SW (1992) Potocytosis: sequestration and transport of small molecules by caveolae. Science 255: 410–411 - PubMed
-
- Beermann C, Wunderli-Allenspach H, Groscurth P, Filgueira L (2000) Lipoproteins from Borrelia burgdorferi applied in liposomes and presented by dendritic cells induce CD8(+) T-lymphocytes in vitro. Cell Immunol 201: 124–131 - PubMed
-
- Bender FC, Reymond MA, Bron C, Quest AF (2000) Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res 60: 5870–5878 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

