Teduglutide ([Gly2]GLP-2) protects small intestinal stem cells from radiation damage
- PMID: 15548172
- PMCID: PMC6495530
- DOI: 10.1111/j.1365-2184.2004.00320.x
Teduglutide ([Gly2]GLP-2) protects small intestinal stem cells from radiation damage
Abstract
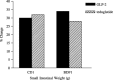
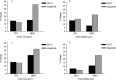
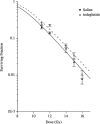
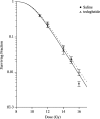
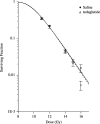
Glucagon-like peptide-2 and its dipeptidyl peptidase (DP-IV) resistant analogue teduglutide are trophic for the gastrointestinal epithelium. Exposure increases villus height and crypt size and results in increased overall intestinal weight. As these effects may be mediated through stimulation of the stem cell compartment, they may promote intestinal healing and act as potential anti-mucositis agents in patients undergoing cancer chemotherapy. A study was initiated to investigate the protective effects of teduglutide on the murine small intestinal epithelium following gamma-irradiation using the crypt microcolony assay as a measure of stem cell survival and functional competence. Teduglutide demonstrated intestinotrophic effects in both CD1 and BDF1 mouse strains. In BDF1 mice, subcutaneous injection of GLP-2 or teduglutide (0.2 mg/kg/day, b.i.d.) for 14 days increased intestinal weight by 28% and resulted in comparable increases in crypt size, villus height and area. Teduglutide given daily for 6 or 14 days prior to whole body, gamma-irradiation significantly increased crypt stem cell survival when compared with vehicle-treated controls. The mean levels of protection over a range of doses provided protection factors from 1.3 to 1.5. A protective effect was only observed when teduglutide was given before irradiation. These results suggest that teduglutide has the ability to modulate clonogenic stem cell survival in the small intestine and this may have a useful clinical application in the prevention of cancer therapy-induced mucositis.
Figures



Similar articles
-
Pretreatment with transforming growth factor beta-3 protects small intestinal stem cells against radiation damage in vivo.Br J Cancer. 1997;75(10):1454-9. doi: 10.1038/bjc.1997.249. Br J Cancer. 1997. PMID: 9166937 Free PMC article.
-
Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients.Gut. 2005 Sep;54(9):1224-31. doi: 10.1136/gut.2004.061440. Gut. 2005. PMID: 16099790 Free PMC article. Clinical Trial.
-
Transforming growth factor-B3 protects murine small intestinal crypt stem cells and animal survival after irradiation, possibly by reducing stem-cell cycling.Int J Cancer. 2000 Apr 1;86(1):53-9. doi: 10.1002/(sici)1097-0215(20000401)86:1<53::aid-ijc8>3.0.co;2-z. Int J Cancer. 2000. PMID: 10728594
-
Teduglutide in intestinal adaptation and repair: light at the end of the tunnel.Expert Opin Investig Drugs. 2008 Jun;17(6):945-51. doi: 10.1517/13543784.17.6.945. Expert Opin Investig Drugs. 2008. PMID: 18491995 Review.
-
Biological actions and therapeutic potential of the glucagon-like peptides.Gastroenterology. 2002 Feb;122(2):531-44. doi: 10.1053/gast.2002.31068. Gastroenterology. 2002. PMID: 11832466 Review.
Cited by
-
Pharmaceutical drugs supporting regeneration of small-intestinal mucosa severely damaged by ionizing radiation in mice.J Radiat Res. 2013 Nov 1;54(6):1057-64. doi: 10.1093/jrr/rrt077. Epub 2013 May 31. J Radiat Res. 2013. PMID: 23728323 Free PMC article.
-
Prevention of Rat Intestinal Injury with a Drug Combination of Melatonin and Misoprostol.Int J Mol Sci. 2020 Sep 15;21(18):6771. doi: 10.3390/ijms21186771. Int J Mol Sci. 2020. PMID: 32942716 Free PMC article.
-
Gut hormones, and short bowel syndrome: the enigmatic role of glucagon-like peptide-2 in the regulation of intestinal adaptation.World J Gastroenterol. 2006 Jul 14;12(26):4117-29. doi: 10.3748/wjg.v12.i26.4117. World J Gastroenterol. 2006. PMID: 16830359 Free PMC article. Review.
-
Inflammation enhances resection-induced intestinal adaptive growth in IL-10 null mice.J Surg Res. 2011 Jun 1;168(1):62-9. doi: 10.1016/j.jss.2009.09.051. Epub 2009 Oct 22. J Surg Res. 2011. PMID: 20074747 Free PMC article.
-
Intestinal stem cell injury and protection during cancer therapy.Transl Cancer Res. 2013 Oct 1;2(5):384-396. Transl Cancer Res. 2013. PMID: 24683536 Free PMC article.
References
-
- Booth D, Haley JD, Bruskin AM, Potten CS (2000) Transforming growth factor β3 protects murine small intestinal crypt stem cells and animal survival after irradiation, possibly by reducing stem cell cycling. Int. J. Cancer 86, 53. - PubMed
-
- Boushey R., Yusta B, Drucker DJ (1999) Glucagon‐like peptide 2 decreases mortality and reduces the severity of indomethacin‐induced murine enteritis. Am. J. Physiol. 277, E937. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

