Exploiting thiol modifications
- PMID: 15547642
- PMCID: PMC526781
- DOI: 10.1371/journal.pbio.0020400
Exploiting thiol modifications
Abstract
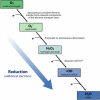
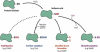
Molecular oxygen may be necessary for life but with its beneficial properties comes formation of potentially toxic reactive oxygen species. One of the ways in which bacteria protect themselves is explained
Figures
Similar articles
-
Interactions between reactive oxygen species and sulfhydryl groups of cysteine, acetylcysteine and glutathione.Pol J Pharmacol. 1995 Jan-Feb;47(1):59-62. Pol J Pharmacol. 1995. PMID: 7550550
-
Indirect electrochemical sensing of radicals and radical scavengers in biological matrices.Angew Chem Int Ed Engl. 2007;46(42):8079-81. doi: 10.1002/anie.200702690. Angew Chem Int Ed Engl. 2007. PMID: 17868166 No abstract available.
-
Thiol modifications in a snapshot.Nat Biotechnol. 2005 Jan;23(1):42-3. doi: 10.1038/nbt0105-42. Nat Biotechnol. 2005. PMID: 15637619 No abstract available.
-
The system biology of thiol redox system in Escherichia coli and yeast: differential functions in oxidative stress, iron metabolism and DNA synthesis.FEBS Lett. 2007 Jul 31;581(19):3598-607. doi: 10.1016/j.febslet.2007.07.002. FEBS Lett. 2007. PMID: 17659286 Review.
-
Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria.Arch Biochem Biophys. 2012 Sep 15;525(2):161-9. doi: 10.1016/j.abb.2012.02.007. Epub 2012 Feb 20. Arch Biochem Biophys. 2012. PMID: 22381957 Review.
Cited by
-
Stress-induced chaperones: a first line of defense against the powerful oxidant hypochlorous acid.F1000Res. 2019 Sep 23;8:F1000 Faculty Rev-1678. doi: 10.12688/f1000research.19517.1. eCollection 2019. F1000Res. 2019. PMID: 31583082 Free PMC article. Review.
-
Posttranslational control of transcription factor FixK2, a key regulator for the Bradyrhizobium japonicum-soybean symbiosis.Proc Natl Acad Sci U S A. 2009 Dec 22;106(51):21860-5. doi: 10.1073/pnas.0908097106. Epub 2009 Dec 2. Proc Natl Acad Sci U S A. 2009. PMID: 19955406 Free PMC article.
-
Shedding light on a Group IV (ECF11) alternative σ factor.Mol Microbiol. 2019 Aug;112(2):374-384. doi: 10.1111/mmi.14280. Epub 2019 May 31. Mol Microbiol. 2019. PMID: 31111523 Free PMC article. Review.
-
Design of Fluorescent Probes for Bioorthogonal Labeling of Carbonylation in Live Cells.Sci Rep. 2020 May 6;10(1):7668. doi: 10.1038/s41598-020-64790-y. Sci Rep. 2020. PMID: 32376913 Free PMC article.
-
Epigenetic Alterations under Oxidative Stress in Stem Cells.Oxid Med Cell Longev. 2022 Aug 29;2022:6439097. doi: 10.1155/2022/6439097. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36071870 Free PMC article. Review.
References
-
- Bae JB, Park JH, Hahn MY, Kim MS, Roe JH. Redox-dependent changes in RsrA, an anti-sigma factor in Streptomyces coelicolor: Zinc release and disulfide bond formation. J Mol Biol. 2004;335:425–435. - PubMed
-
- Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. - PubMed
-
- Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. - PubMed
-
- Collet JF, D'Souza JC, Jakob U, Bardwell JC. Thioredoxin 2, an oxidative stress-induced protein, contains a high affinity zinc binding site. J Biol Chem. 2003;278:45325–45332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

