Nuclear speckle-associated protein Pnn/DRS binds to the transcriptional corepressor CtBP and relieves CtBP-mediated repression of the E-cadherin gene
- PMID: 15542832
- PMCID: PMC529029
- DOI: 10.1128/MCB.24.23.10223-10235.2004
Nuclear speckle-associated protein Pnn/DRS binds to the transcriptional corepressor CtBP and relieves CtBP-mediated repression of the E-cadherin gene
Abstract
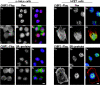
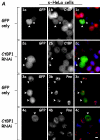
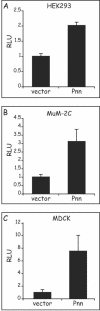
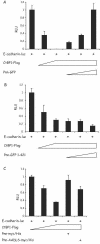
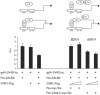
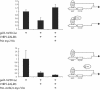
Previously, we have shown that pinin/DRS (Pnn), a 140-kDa nuclear and cell adhesion-related phosphoprotein, is involved in the regulation of cell adhesion and modulation of the activity of multiple tumor suppressor genes. In the nucleus Pnn is concentrated in the "nuclear speckles," zones of accumulation of transcriptional and mRNA splicing factors, where Pnn is involved in mRNA processing. Alternatively, other roles of Pnn in gene regulation have not yet been established. By utilizing in vitro pull-down assays, in vivo interaction studies, and immunofluorescence in combination with overexpression and RNA interference experiments, we present evidence that Pnn interacts with the known transcriptional corepressor CtBP1. As a consequence of this interaction Pnn was capable of relieving the CtBP1-mediated repression of E-cadherin promoter activity. Our results suggest that the interaction of Pnn with the corepressor CtBP1 may modulate repression of transcription by CtBP1. This interaction may reflect the existence of coupling factors involved in CtBP-mediated transcriptional regulation and mRNA processing events.
Figures



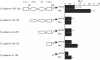
Similar articles
-
Corepressor CtBP and nuclear speckle protein Pnn/DRS differentially modulate transcription and splicing of the E-cadherin gene.Mol Cell Biol. 2008 Mar;28(5):1584-95. doi: 10.1128/MCB.00421-07. Epub 2007 Dec 17. Mol Cell Biol. 2008. PMID: 18086895 Free PMC article.
-
Interaction between Smad-interacting protein-1 and the corepressor C-terminal binding protein is dispensable for transcriptional repression of E-cadherin.J Biol Chem. 2003 Jul 11;278(28):26135-45. doi: 10.1074/jbc.M300597200. Epub 2003 Apr 24. J Biol Chem. 2003. PMID: 12714599
-
Reduction of Pnn by RNAi induces loss of cell-cell adhesion between human corneal epithelial cells.Mol Vis. 2005 Feb 18;11:133-42. Mol Vis. 2005. PMID: 15735603
-
Transcriptional regulation by C-terminal binding proteins.Int J Biochem Cell Biol. 2007;39(9):1593-607. doi: 10.1016/j.biocel.2007.01.025. Epub 2007 Feb 4. Int J Biochem Cell Biol. 2007. PMID: 17336131 Review.
-
The role and regulation of Pnn in proliferative and non-dividing cells: Form embryogenesis to pathogenesis.Biochem Pharmacol. 2021 Oct;192:114672. doi: 10.1016/j.bcp.2021.114672. Epub 2021 Jul 5. Biochem Pharmacol. 2021. PMID: 34237338 Review.
Cited by
-
Pinin Induces Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma by Regulating m6A Modification.J Oncol. 2021 Dec 7;2021:7529164. doi: 10.1155/2021/7529164. eCollection 2021. J Oncol. 2021. PMID: 34917148 Free PMC article.
-
Toosendanin Restrains Idiopathic Pulmonary Fibrosis by Inhibiting ZEB1/CTBP1 Interaction.Curr Mol Med. 2024;24(1):123-133. doi: 10.2174/1566524023666230501205149. Curr Mol Med. 2024. PMID: 37138491 Free PMC article.
-
The prognostic effect of PNN in digestive tract cancers and its correlation with the tumor immune landscape in colon adenocarcinoma.J Clin Lab Anal. 2022 Apr;36(4):e24327. doi: 10.1002/jcla.24327. Epub 2022 Mar 8. J Clin Lab Anal. 2022. PMID: 35257416 Free PMC article.
-
Mechanisms directing the nuclear localization of the CtBP family proteins.Mol Cell Biol. 2006 Jul;26(13):4882-94. doi: 10.1128/MCB.02402-05. Mol Cell Biol. 2006. PMID: 16782877 Free PMC article.
-
Role of the C-terminal binding protein PXDLS motif binding cleft in protein interactions and transcriptional repression.Mol Cell Biol. 2006 Nov;26(21):8202-13. doi: 10.1128/MCB.00445-06. Epub 2006 Aug 28. Mol Cell Biol. 2006. PMID: 16940173 Free PMC article.
References
-
- Barnes, C. J., R. K. Vadlamudi, S. K. Mishra, R. H. Jacobson, F. Li, and R. Kumar. 2003. Functional inactivation of a transcriptional corepressor by a signaling kinase. Nat. Struct. Biol. 10:622-628. - PubMed
-
- Bellavite, P., F. Bazzoni, M. A. Cassatella, K. J. Hunter, and J. V. Bannister. 1990. Isolation and characterization of a cDNA clone for a novel serine-rich neutrophil protein. Biochem. Biophys. Res. Commun. 170:915-922. - PubMed
-
- Boyd, J. M., T. Subramanian, U. Schaeper, M. La Regina, S. Bayley, and G. Chinnadurai. 1993. A region in the C terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis, and metastasis. EMBO J. 12:469-478. - PMC - PubMed
-
- Brandner, J. M., S. Reidenbach, and W. W. Franke. 1997. Evidence that “pinin,” reportedly a differentiation-specific desmosomal protein, is actually a widespread nuclear protein. Differentiation 62:119-127. - PubMed
-
- Burke, L. J., and A. Bahianmad. 2000. Corepressors 2000. FASEB J. 14:1876-1888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
