Inhibition of human immunodeficiency virus replication by a dual CCR5/CXCR4 antagonist
- PMID: 15542651
- PMCID: PMC524989
- DOI: 10.1128/JVI.78.23.12996-13006.2004
Inhibition of human immunodeficiency virus replication by a dual CCR5/CXCR4 antagonist
Abstract
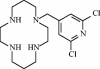
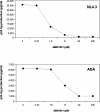
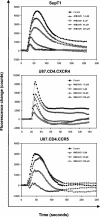
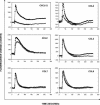
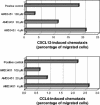
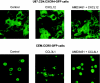
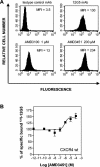
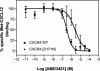
Here we report that the N-pyridinylmethyl cyclam analog AMD3451 has antiviral activity against a wide variety of R5, R5/X4, and X4 strains of human immunodeficiency virus type 1 (HIV-1) and HIV-2 (50% inhibitory concentration [IC(50)] ranging from 1.2 to 26.5 microM) in various T-cell lines, CCR5- or CXCR4-transfected cells, peripheral blood mononuclear cells (PBMCs), and monocytes/macrophages. AMD3451 also inhibited R5, R5/X4, and X4 HIV-1 primary clinical isolates in PBMCs (IC(50), 1.8 to 7.3 microM). A PCR-based viral entry assay revealed that AMD3451 blocks R5 and X4 HIV-1 infection at the virus entry stage. AMD3451 dose-dependently inhibited the intracellular Ca(2+) signaling induced by the CXCR4 ligand CXCL12 in T-lymphocytic cells and in CXCR4-transfected cells, as well as the Ca(2+) flux induced by the CCR5 ligands CCL5, CCL3, and CCL4 in CCR5-transfected cells. The compound did not interfere with chemokine-induced Ca(2+) signaling through CCR1, CCR2, CCR3, CCR4, CCR6, CCR9, or CXCR3 and did not induce intracellular Ca(2+) signaling by itself at concentrations up to 400 microM. In freshly isolated monocytes, AMD3451 inhibited the Ca(2+) flux induced by CXCL12 and CCL4 but not that induced by CCL2, CCL3, CCL5, and CCL7. The CXCL12- and CCL3-induced chemotaxis was also dose-dependently inhibited by AMD3451. Furthermore, AMD3451 inhibited CXCL12- and CCL3L1-induced endocytosis in CXCR4- and CCR5-transfected cells. AMD3451, in contrast to the specific CXCR4 antagonist AMD3100, did not inhibit but enhanced the binding of several anti-CXCR4 monoclonal antibodies (such as clone 12G5) at the cell surface, pointing to a different interaction with CXCR4. AMD3451 is the first low-molecular-weight anti-HIV agent with selective HIV coreceptor, CCR5 and CXCR4, interaction.
Figures

Similar articles
-
AMD3465, a monomacrocyclic CXCR4 antagonist and potent HIV entry inhibitor.Biochem Pharmacol. 2005 Sep 1;70(5):752-61. doi: 10.1016/j.bcp.2005.05.035. Biochem Pharmacol. 2005. PMID: 16011832
-
Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization.J Leukoc Biol. 2003 Dec;74(6):1074-82. doi: 10.1189/jlb.0203067. Epub 2003 Sep 12. J Leukoc Biol. 2003. PMID: 12972507
-
Cyclic zinc-dithiocarbamate-S,S'-dioxide blocks CXCR4-mediated HIV-1 infection.Biochem Biophys Res Commun. 2000 Jun 7;272(2):351-6. doi: 10.1006/bbrc.2000.2779. Biochem Biophys Res Commun. 2000. PMID: 10833417
-
HIV co-receptors as targets for antiviral therapy.Curr Top Med Chem. 2004;4(9):883-93. doi: 10.2174/1568026043388501. Curr Top Med Chem. 2004. PMID: 15134547 Review.
-
HIV chemokine receptor inhibitors as novel anti-HIV drugs.Cytokine Growth Factor Rev. 2005 Dec;16(6):659-77. doi: 10.1016/j.cytogfr.2005.05.009. Epub 2005 Jul 6. Cytokine Growth Factor Rev. 2005. PMID: 16005254 Review.
Cited by
-
C6-C8 bridged epothilones: consequences of installing a conformational lock at the edge of the macrocycle.Chemistry. 2011 Dec 23;17(52):14792-804. doi: 10.1002/chem.201102630. Epub 2011 Nov 30. Chemistry. 2011. PMID: 22127984 Free PMC article.
-
Pyrazolo-Piperidines Exhibit Dual Inhibition of CCR5/CXCR4 HIV Entry and Reverse Transcriptase.ACS Med Chem Lett. 2015 May 6;6(7):753-7. doi: 10.1021/acsmedchemlett.5b00036. eCollection 2015 Jul 9. ACS Med Chem Lett. 2015. PMID: 26191361 Free PMC article.
-
Agarose Spot as a Comparative Method for in situ Analysis of Simultaneous Chemotactic Responses to Multiple Chemokines.Sci Rep. 2017 Apr 21;7(1):1075. doi: 10.1038/s41598-017-00949-4. Sci Rep. 2017. PMID: 28432337 Free PMC article.
-
Comprehensive Transcriptomic Comparison between Porcine CD8- and CD8+ Gamma Delta T Cells Revealed Distinct Immune Phenotype.Animals (Basel). 2021 Jul 22;11(8):2165. doi: 10.3390/ani11082165. Animals (Basel). 2021. PMID: 34438623 Free PMC article.
-
CXCR7/ACKR3-targeting ligands interfere with X7 HIV-1 and HIV-2 entry and replication in human host cells.Heliyon. 2018 Mar 1;4(3):e00557. doi: 10.1016/j.heliyon.2018.e00557. eCollection 2018 Mar. Heliyon. 2018. PMID: 29560468 Free PMC article.
References
-
- Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. - PubMed
-
- Aquaro, S., O. Wedgwood, C. Yarnold, D. Cahard, R. Pathinara, C. McGuigan, R. Calio, E. De Clercq, J. Balzarini, and C. F. Perno. 2000. Activities of masked 2′,3′-dideoxynucleoside monophosphate derivatives against human immunodeficiency virus in resting macrophages. Antimicrob. Agents Chemother. 44:173-177. - PMC - PubMed
-
- Arenzana-Seisdedos, F., J. L. Virelizier, D. Rousset, I. Clark-Lewis, P. Loetscher, B. Moser, and M. Baggiolini. 1996. HIV blocked by chemokine antagonist. Nature 383:400. - PubMed
-
- Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

