Altered endochondral bone development in matrix metalloproteinase 13-deficient mice
- PMID: 15539485
- PMCID: PMC2771178
- DOI: 10.1242/dev.01461
Altered endochondral bone development in matrix metalloproteinase 13-deficient mice
Abstract
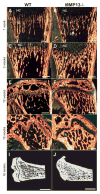
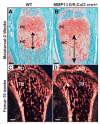
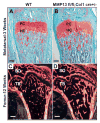
The assembly and degradation of extracellular matrix (ECM) molecules are crucial processes during bone development. In this study, we show that ECM remodeling is a critical rate-limiting step in endochondral bone formation. Matrix metalloproteinase (MMP) 13 (collagenase 3) is poised to play a crucial role in bone formation and remodeling because of its expression both in terminal hypertrophic chondrocytes in the growth plate and in osteoblasts. Moreover, a mutation in the human MMP13 gene causes the Missouri variant of spondyloepimetaphyseal dysplasia. Inactivation of Mmp13 in mice through homologous recombination led to abnormal skeletal growth plate development. Chondrocytes differentiated normally but their exit from the growth plate was delayed. The severity of the Mmp13- null growth plate phenotype increased until about 5 weeks and completely resolved by 12 weeks of age. Mmp13-null mice had increased trabecular bone, which persisted for months. Conditional inactivation of Mmp13 in chondrocytes and osteoblasts showed that increases in trabecular bone occur independently of the improper cartilage ECM degradation caused by Mmp13 deficiency in late hypertrophic chondrocytes. Our studies identified the two major components of the cartilage ECM, collagen type II and aggrecan, as in vivo substrates for MMP13. We found that degradation of cartilage collagen and aggrecan is a coordinated process in which MMP13 works synergistically with MMP9. Mice lacking both MMP13 and MMP9 had severely impaired endochondral bone, characterized by diminished ECM remodeling, prolonged chondrocyte survival, delayed vascular recruitment and defective trabecular bone formation (resulting in drastically shortened bones). These data support the hypothesis that proper ECM remodeling is the dominant rate-limiting process for programmed cell death, angiogenesis and osteoblast recruitment during normal skeletal morphogenesis.
Figures






Similar articles
-
Galectin-3 is a downstream regulator of matrix metalloproteinase-9 function during endochondral bone formation.Mol Biol Cell. 2005 Jun;16(6):3028-39. doi: 10.1091/mbc.e04-12-1119. Epub 2005 Mar 30. Mol Biol Cell. 2005. PMID: 15800063 Free PMC article.
-
Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification.J Biol Chem. 2005 May 13;280(19):19185-95. doi: 10.1074/jbc.M414275200. Epub 2005 Mar 10. J Biol Chem. 2005. PMID: 15760903
-
Complementary interplay between matrix metalloproteinase-9, vascular endothelial growth factor and osteoclast function drives endochondral bone formation.Dis Model Mech. 2010 Mar-Apr;3(3-4):224-35. doi: 10.1242/dmm.004226. Epub 2010 Feb 8. Dis Model Mech. 2010. PMID: 20142327 Free PMC article.
-
Cartilage matrix resorption in skeletogenesis.Novartis Found Symp. 2001;232:158-66; discussion 166-70. doi: 10.1002/0470846658.ch11. Novartis Found Symp. 2001. PMID: 11277078 Review.
-
How proteases regulate bone morphogenesis.Ann N Y Acad Sci. 2003 May;995:109-16. doi: 10.1111/j.1749-6632.2003.tb03214.x. Ann N Y Acad Sci. 2003. PMID: 12814943 Review.
Cited by
-
Regulation of the Bioavailability of TGF-β and TGF-β-Related Proteins.Cold Spring Harb Perspect Biol. 2016 Jun 1;8(6):a021907. doi: 10.1101/cshperspect.a021907. Cold Spring Harb Perspect Biol. 2016. PMID: 27252363 Free PMC article. Review.
-
Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance.J Bone Miner Res. 2012 Sep;27(9):1936-50. doi: 10.1002/jbmr.1646. J Bone Miner Res. 2012. PMID: 22549931 Free PMC article.
-
Deficiency of matrix metalloproteinase-13 increases inflammation after acute lung injury.Exp Lung Res. 2010 Dec;36(10):615-24. doi: 10.3109/01902148.2010.497201. Epub 2010 Sep 23. Exp Lung Res. 2010. PMID: 20860538 Free PMC article.
-
Parathyroid hormone activation of matrix metalloproteinase-13 transcription requires the histone acetyltransferase activity of p300 and PCAF and p300-dependent acetylation of PCAF.J Biol Chem. 2010 Dec 3;285(49):38014-22. doi: 10.1074/jbc.M110.142141. Epub 2010 Sep 24. J Biol Chem. 2010. PMID: 20870727 Free PMC article.
-
Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering.Proc Natl Acad Sci U S A. 2010 Apr 20;107(16):7251-6. doi: 10.1073/pnas.1000302107. Epub 2010 Apr 6. Proc Natl Acad Sci U S A. 2010. PMID: 20406908 Free PMC article.
References
-
- Abbaszade I, Liu RQ, Yang F, Rosenfeld SA, Ross OH, Link JR, Ellis DM, Tortorella MD, Pratta MA, Hollis JM, et al. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999;274:23443–23450. - PubMed
-
- Albrecht U, Eichele G, Helms JA, Lu H. Visualization of gene expression patterns by in situ hybridization. In: Daston G, editor. Molecular and Cellular Methods in Developmental Toxicology. Boca Raton, FL: CRC Press; 1997. pp. 23–48.
-
- Alini M, Matsui Y, Dodge GR, Poole AR. The extracellular matrix of cartilage in the growth plate before and during calcification: changes in composition and degradation of type II collagen. Calcif Tissue Int. 1992;50:327–335. - PubMed
-
- Arner EC. Aggrecanase-mediated cartilage degradation. Curr Opin Pharmacol. 2002;2:322–329. - PubMed
-
- Aszodi A, Bateman JF, Gustafsson E, Boot-Handford R, Fassler R. Mammalian skeletogenesis and extracellular matrix: what can we learn from knockout mice? Cell Struct Funct. 2000;25:73–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

