Improved behavior and neuropathology in the mouse model of Sanfilippo type IIIB disease after adeno-associated virus-mediated gene transfer in the striatum
- PMID: 15537895
- PMCID: PMC6730192
- DOI: 10.1523/JNEUROSCI.3558-04.2004
Improved behavior and neuropathology in the mouse model of Sanfilippo type IIIB disease after adeno-associated virus-mediated gene transfer in the striatum
Abstract
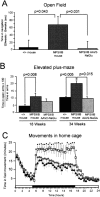
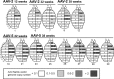
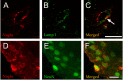
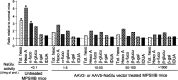
Sanfilippo syndrome is a mucopolysaccharidosis (MPS) caused by a lysosomal enzyme defect interrupting the degradation pathway of heparan sulfates. Affected children develop hyperactivity, aggressiveness, delayed development, and severe neuropathology. We observed relevant behaviors in the mouse model of Sanfilippo syndrome type B (MPSIIIB), in which the gene coding for alpha-N-acetylglucosaminidase (NaGlu) is invalidated. We addressed the feasibility of gene therapy in these animals. Vectors derived from adeno-associated virus serotype 2 (AAV2) or 5 (AAV5) coding for NaGlu were injected at a single site in the putamen of 45 6-week-old MPSIIIB mice. Normal behavior was observed in treated mice. High NaGlu activity, far above physiological levels, was measured in the brain and persisted at 38 weeks of age. NaGlu immunoreactivity was detected in neuron intracellular organelles, including lysosomes. Enzyme activity spread beyond vector diffusion areas. Delivery to the entire brain was reproducibly obtained with both vector types. NaGlu activity was higher and distribution was broader with AAV5-NaGlu than with AAV2-NaGlu vectors. The compensatory increase in the activity of various lysosomal enzymes was improved. The accumulation of gangliosides GM2 and GM3 present before treatment and possibly participating in neuropathology was reversed. Characteristic vacuolations in microglia, perivascular cells, and neurons, which were prominent before the age of treatment, disappeared in areas in which NaGlu was present. However, improvement was only partial in some animals, in contrast to high NaGlu activity. These results indicate that NaGlu delivery from intracerebral sources has the capacity to alleviate most disease manifestations in the MPSIIIB mouse model.
Figures



Similar articles
-
Neurological correction of lysosomal storage in a mucopolysaccharidosis IIIB mouse model by adeno-associated virus-mediated gene delivery.Mol Ther. 2002 Jan;5(1):42-9. doi: 10.1006/mthe.2001.0514. Mol Ther. 2002. PMID: 11786044
-
Restoration of central nervous system alpha-N-acetylglucosaminidase activity and therapeutic benefits in mucopolysaccharidosis IIIB mice by a single intracisternal recombinant adeno-associated viral type 2 vector delivery.J Gene Med. 2010 Jul;12(7):624-33. doi: 10.1002/jgm.1480. J Gene Med. 2010. PMID: 20603889
-
Disease correction by combined neonatal intracranial AAV and systemic lentiviral gene therapy in Sanfilippo Syndrome type B mice.Gene Ther. 2013 Sep;20(9):913-21. doi: 10.1038/gt.2013.14. Epub 2013 Mar 28. Gene Ther. 2013. PMID: 23535899 Free PMC article.
-
In Vivo Gene Therapy for Mucopolysaccharidosis Type III (Sanfilippo Syndrome): A New Treatment Horizon.Hum Gene Ther. 2019 Oct;30(10):1211-1221. doi: 10.1089/hum.2019.217. Hum Gene Ther. 2019. PMID: 31482754 Review.
-
Molecular genetics of mucopolysaccharidosis type IIIA and IIIB: Diagnostic, clinical, and biological implications.Hum Mutat. 2001 Oct;18(4):264-81. doi: 10.1002/humu.1189. Hum Mutat. 2001. PMID: 11668611 Review.
Cited by
-
Genistein improves neuropathology and corrects behaviour in a mouse model of neurodegenerative metabolic disease.PLoS One. 2010 Dec 1;5(12):e14192. doi: 10.1371/journal.pone.0014192. PLoS One. 2010. PMID: 21152017 Free PMC article.
-
Gene therapy for neurologic manifestations of mucopolysaccharidoses.Expert Opin Drug Deliv. 2015 Feb;12(2):283-96. doi: 10.1517/17425247.2015.966682. Epub 2014 Dec 16. Expert Opin Drug Deliv. 2015. PMID: 25510418 Free PMC article. Review.
-
Temporospatial Development of Neuropathologic Findings in a Canine Model of Mucopolysaccharidosis IIIB.Vet Pathol. 2021 Jan;58(1):205-222. doi: 10.1177/0300985820960128. Epub 2020 Nov 18. Vet Pathol. 2021. PMID: 33205707 Free PMC article.
-
A next step in adeno-associated virus-mediated gene therapy for neurological diseases: regulation and targeting.Br J Clin Pharmacol. 2013 Aug;76(2):217-32. doi: 10.1111/bcp.12065. Br J Clin Pharmacol. 2013. PMID: 23331189 Free PMC article. Review.
-
Gene Therapy for Lysosomal Storage Disorders: Ongoing Studies and Clinical Development.Biomolecules. 2021 Apr 20;11(4):611. doi: 10.3390/biom11040611. Biomolecules. 2021. PMID: 33924076 Free PMC article. Review.
References
-
- Adra CN, Boer PH, McBurney M (1987) Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene 60: 65-74. - PubMed
-
- Avale ME, Falzone TL, Gelman DM, Low MJ, Grandy DK, Rubinstein M (2004) The dopamine D4 receptor is essential for hyperactivity and impaired behavioral inhibition in a mouse model of attention deficit/hyperactivity disorder. Mol Psychiatry 9: 718-726. - PubMed
-
- Bhaumik M, Muller VJ, Rozaklis T, Johnson L, Dobrenis K, Bhattacharyya R, Wurzelmann S, Finamore P, Hopwood JJ, Walkley SU, Stanley P (1999) A mouse model for mucopolysaccharidosis type III A (Sanfilippo syndrome). Glycobiology 9: 1389-1396. - PubMed
-
- Bosch A, Heard JM (2003) Gene therapy for mucopolysaccharidosis. Int Rev Neurobiol 55: 271-296. - PubMed
-
- Brument N, Morenweiser R, Blouin V, Toublanc E, Raimbaud I, Chérel Y, Folliot S, Gaden F, Boulanger P, Kroner-Lux G, Moullier P, Rolling F, Salvetti A (2002) A versatile and scalable two-step ion-exchange chromatography process for the purification of recombinant adeno-associated virus serotypes-2 and -5. Mol Ther 6: 678-686. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
