Stimulation of beta-adrenoceptors inhibits store-operated channel currents via a cAMP-dependent protein kinase mechanism in rabbit portal vein myocytes
- PMID: 15528235
- PMCID: PMC1665505
- DOI: 10.1113/jphysiol.2004.077602
Stimulation of beta-adrenoceptors inhibits store-operated channel currents via a cAMP-dependent protein kinase mechanism in rabbit portal vein myocytes
Abstract
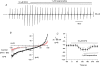
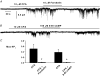
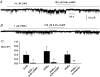
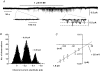
Previously we have described the properties of store-operated channel currents (SOCs) in freshly dispersed rabbit portal vein smooth muscle cells. In addition to Ca(2+) store depletion these SOCs could also be activated by alpha-adrenoceptor stimulation and diacylglycerol (DAG) via a protein kinase C (PKC)-dependent mechanism. In the present study we have investigated the effect of beta-adrenoceptor stimulation on SOCs in rabbit portal vein myocytes. With whole-cell recording the selective beta-adrenoceptor agonist isoprenaline reduced the current evoked by cyclopiazonic acid (CPA, sarcoplasmic/endoplasmic reticulum ATPase inhibitor) by over 85%. With cell-attached patch recording, bath application of isoprenaline produced a pronounced inhibition of SOC activity evoked by either CPA or the acetoxymethyl ester form of BAPTA (BAPTA-AM). SOC activity evoked by CPA, the DAG analogue, 1-oleoyl-acetyl-sn-glycerol (OAG) or the phorbol ester, phorbol-12,13-dibutyrate (PDBu) was also markedly inhibited by the adenylate cyclase activator, forskolin, and the cell-permeable non-hydrolysable analogue of cyclic adenosine monophosphate (cAMP), 8-Br-cAMP. With inside-out patches, bath application of PDBu evoked channel currents with similar properties to SOCs which were inhibited by over 90% by a catalytic subunit of protein kinase A (PKA) and by 8-Br-cAMP. Moreover bath application of PKA inhibitors, H-89, KT5720 and an inhibitory peptide to quiescent cell-attached or inside-out patches, activated channel currents with similar properties to SOCs. These data suggest that in rabbit portal vein myocytes, stimulation of beta-adrenoceptors inhibits SOC activity via a cAMP-dependent protein kinase signal transduction cascade. In addition it is concluded that constitutive PKA activity has a profound inhibitory effect on SOC activity in this vascular preparation.
Figures


Similar articles
-
Dual effect of calmodulin on store-operated Ca2+ -permeable cation channels in rabbit portal vein myocytes.Br J Pharmacol. 2006 Aug;148(7):1001-11. doi: 10.1038/sj.bjp.0706797. Epub 2006 Jun 12. Br J Pharmacol. 2006. PMID: 16770321 Free PMC article.
-
Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes.J Physiol. 2002 Oct 1;544(Pt 1):113-25. doi: 10.1113/jphysiol.2002.022574. J Physiol. 2002. PMID: 12356885 Free PMC article.
-
Facilitatory effect of Ins(1,4,5)P3 on store-operated Ca2+-permeable cation channels in rabbit portal vein myocytes.J Physiol. 2005 Jul 1;566(Pt 1):161-71. doi: 10.1113/jphysiol.2005.088260. Epub 2005 Apr 28. J Physiol. 2005. PMID: 15860523 Free PMC article.
-
Regulation of 4-aminopyridine-sensitive, delayed rectifier K+ channels in vascular smooth muscle by phosphorylation.Biochem Cell Biol. 1996;74(4):439-47. doi: 10.1139/o96-048. Biochem Cell Biol. 1996. PMID: 8960350 Review.
-
Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells.J Physiol. 2007 Aug 15;583(Pt 1):25-36. doi: 10.1113/jphysiol.2007.137802. Epub 2007 Jul 5. J Physiol. 2007. PMID: 17615095 Free PMC article. Review.
Cited by
-
Dual effect of calmodulin on store-operated Ca2+ -permeable cation channels in rabbit portal vein myocytes.Br J Pharmacol. 2006 Aug;148(7):1001-11. doi: 10.1038/sj.bjp.0706797. Epub 2006 Jun 12. Br J Pharmacol. 2006. PMID: 16770321 Free PMC article.
-
Caffeine inhibits InsP3 responses and capacitative calcium entry in canine pulmonary arterial smooth muscle cells.Vascul Pharmacol. 2009 Mar-Apr;50(3-4):89-97. doi: 10.1016/j.vph.2008.11.001. Epub 2008 Nov 21. Vascul Pharmacol. 2009. PMID: 19084078 Free PMC article.
-
Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes.J Physiol. 2006 Dec 1;577(Pt 2):479-95. doi: 10.1113/jphysiol.2006.119305. Epub 2006 Sep 14. J Physiol. 2006. PMID: 16973707 Free PMC article.
-
Diverse properties of store-operated TRPC channels activated by protein kinase C in vascular myocytes.J Physiol. 2008 May 15;586(10):2463-76. doi: 10.1113/jphysiol.2008.152157. Epub 2008 Mar 20. J Physiol. 2008. PMID: 18356201 Free PMC article.
-
Protein kinases modulate store-operated channels in pulmonary artery smooth muscle cells.J Biomed Sci. 2011 Jan 6;18(1):2. doi: 10.1186/1423-0127-18-2. J Biomed Sci. 2011. PMID: 21211029 Free PMC article.
References
-
- Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase Cα phosphorylates transient receptor potential channel-1 (TRPC1) and regulates store-operated Ca2+ entry. Role in signalling increased endothelial permeability. J Biol Chem. 2004;279:20941–20949. 10.1074/jbc.M313975200. - DOI - PubMed
-
- Albert AP, Large WA. A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes. J Physiol. 2002a;538:717–728. 10.1113/jphysiol.2001.013101. - DOI - PMC - PubMed
-
- Albert AP, Large WA. Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes. J Physiol. 2002b;544:113–125. 10.1113/jphysiol.2002.022574. - DOI - PMC - PubMed
-
- Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium. 2003;33:345–356. 10.1016/S0143-4160(03)00048-4. - DOI - PubMed
-
- Colquhoun D. Practical analysis of single channel records. In: Standen NB, Gray PTA, Whitaker MJ, editors. Microelectrode Techniques. Cambridge: The Company of Biologists; 1987. pp. 83–104.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

