The kinetic profile of intracellular calcium predicts long-term potentiation and long-term depression
- PMID: 15525769
- PMCID: PMC6730235
- DOI: 10.1523/JNEUROSCI.0738-04.2004
The kinetic profile of intracellular calcium predicts long-term potentiation and long-term depression
Abstract
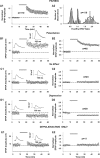
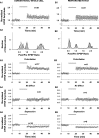
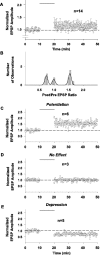
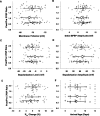
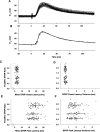
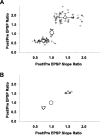
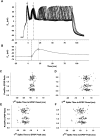
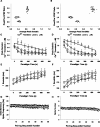
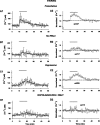
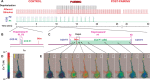
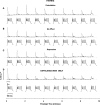
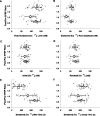
Efficiency of synaptic transmission within the neocortex is regulated throughout life by experience and activity. Periods of correlated or uncorrelated presynaptic and postsynaptic activity lead to enduring changes in synaptic efficiency [long-term potentiation (LTP) and long-term depression (LTD), respectively]. The initial plasticity triggering event is thought to be a precipitous rise in postsynaptic intracellular calcium, with higher levels inducing LTP and more moderate levels inducing LTD. We used a pairing protocol in visual cortical brain slices from young guinea pigs with whole-cell recording and calcium imaging to compare the kinetic profiles of calcium signals generated in response to individual pairings along with the cumulative calcium wave and plasticity outcome. The identical pairing protocol applied to layer 2/3 pyramidal neurons results in different plasticity outcomes between cells. These differences are not attributable to variations in the conditioning protocol, cellular properties, inter-animal variability, animal age, differences in spike timing between the synaptic response and spikes, washout of plasticity factors, recruitment of inhibition, or activation of different afferents. The different plasticity outcomes are reliably predicted by individual intracellular calcium transients in the dendrites after the first few pairings. In addition to the differences in the individual calcium transients, the cumulative calcium wave that spreads to the soma also has a different profile for cells that undergo LTP versus LTD. We conclude that there are biological differences between like-type cells in the dendritic calcium signals generated by coincident synaptic input and spiking that determine the sign of the plasticity response after brief associations.
Figures
Similar articles
-
Relation between dendritic Ca2+ levels and the polarity of synaptic long-term modifications in rat visual cortex neurons.Eur J Neurosci. 1997 Nov;9(11):2309-22. doi: 10.1111/j.1460-9568.1997.tb01648.x. Eur J Neurosci. 1997. PMID: 9464925
-
Long-term depression in rat visual cortex is associated with a lower rise of postsynaptic calcium than long-term potentiation.Neurosci Res. 1996 Feb;24(3):265-74. doi: 10.1016/0168-0102(95)01001-7. Neurosci Res. 1996. PMID: 8815446
-
Photolysis of postsynaptic caged Ca2+ can potentiate and depress mossy fiber synaptic responses in rat hippocampal CA3 pyramidal neurons.J Neurophysiol. 2004 Apr;91(4):1596-607. doi: 10.1152/jn.01073.2003. Epub 2003 Nov 26. J Neurophysiol. 2004. PMID: 14645386 Free PMC article.
-
Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity.Nat Neurosci. 2005 Jul;8(7):839-41. doi: 10.1038/nn0705-839. Nat Neurosci. 2005. PMID: 16136666 Review.
-
The role of dendritic filtering in associative long-term synaptic plasticity.Learn Mem. 1999 Sep-Oct;6(5):422-47. doi: 10.1101/lm.6.5.422. Learn Mem. 1999. PMID: 10541464 Review.
Cited by
-
Plasticity of horizontal connections at a functional border in adult rat somatosensory cortex.Neural Plast. 2009;2009:294192. doi: 10.1155/2009/294192. Epub 2010 Mar 3. Neural Plast. 2009. PMID: 20204080 Free PMC article.
-
STDP in a bistable synapse model based on CaMKII and associated signaling pathways.PLoS Comput Biol. 2007 Nov;3(11):e221. doi: 10.1371/journal.pcbi.0030221. Epub 2007 Sep 26. PLoS Comput Biol. 2007. PMID: 18052535 Free PMC article.
-
Deep-prior ODEs augment fluorescence imaging with chemical sensors.Nat Commun. 2024 Oct 24;15(1):9172. doi: 10.1038/s41467-024-53232-2. Nat Commun. 2024. PMID: 39448575 Free PMC article.
-
A spike-timing-dependent plasticity rule for dendritic spines.Nat Commun. 2020 Aug 26;11(1):4276. doi: 10.1038/s41467-020-17861-7. Nat Commun. 2020. PMID: 32848151 Free PMC article.
-
Pull-push neuromodulation of LTP and LTD enables bidirectional experience-induced synaptic scaling in visual cortex.Neuron. 2012 Feb 9;73(3):497-510. doi: 10.1016/j.neuron.2011.11.023. Neuron. 2012. PMID: 22325202 Free PMC article.
References
-
- Abraham WC, Bear MF (1996) Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126-130. - PubMed
-
- Artola A, Singer W (1993) Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci 16: 480-487. - PubMed
-
- Artola A, Brocher S, Singer W (1990) Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature 347: 69-72. - PubMed
-
- Bear MF, Cooper LN, Ebner FF (1987) A physiological basis for a theory of synapse modification. Science 237: 42-48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
