The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia-reperfusion injury
- PMID: 15509537
- PMCID: PMC1618654
- DOI: 10.1016/S0002-9440(10)63424-4
The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia-reperfusion injury
Abstract
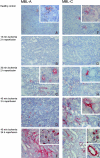
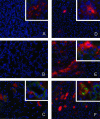
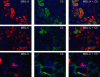
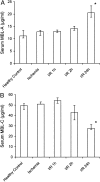
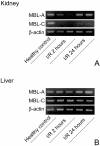
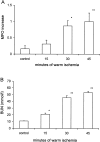
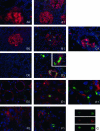
Ischemia-reperfusion (I/R) is an important cause of acute renal failure (ARF). The complement system appears to be essentially involved in I/R injury. However, via which pathway the complement system is activated and in particular whether the mannose-binding lectin (MBL)-pathway is activated is unclear. This tempted us to study the activation and regulation of the MBL-pathway in the course of experimental renal I/R injury and in clinical post-transplant ARF. Mice subjected to renal I/R displayed evident renal MBL-depositions, depending on the duration of warm ischemia, in the early reperfusion phase. Renal deposition of C3, C6 and C9 was observed in the later reperfusion phase. The deposition of MBL-A and -C completely co-localized with the late complement factor C6, showing that MBL is involved in complement activation in the course of renal I/R injury. Moreover, the degree of early MBL-deposition correlated with complement activation, neutrophil-influx, and organ-failure observed in the later reperfusion phase. In serum of mice subjected to renal I/R MBL-A, levels increased in contrast to MBL-C levels, which dropped evidently. In line, liver mRNA levels for MBL-A increased, whereas MBL-C levels decreased. Renal MBL mRNA levels rapidly dropped in the course of renal I/R. Finally, in human biopsies, MBL-depositions were observed early after transplantation of ischemically injured kidneys. In line with our experimental data, in ischemically injured grafts displaying post-transplant organ-failure extensive MBL depositions were observed in peritubular capillaries and tubular epithelial cells. In conclusion, in experimental renal I/R injury and clinical post-transplant ARF the MBL-pathway is activated, followed by activation of the complement system. These data indicate that the MBL-pathway is involved in ischemia-induced complement activation.
Figures
Similar articles
-
Mannan-binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury.Scand J Immunol. 2005 May;61(5):426-34. doi: 10.1111/j.1365-3083.2005.01591.x. Scand J Immunol. 2005. PMID: 15882434
-
Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: inhibition of late apoptosis and inflammation.Transplantation. 2003 Feb 15;75(3):375-82. doi: 10.1097/01.TP.0000044455.05584.2A. Transplantation. 2003. PMID: 12589162
-
Activation of the lectin pathway of complement in pig-to-human xenotransplantation models.Transplantation. 2013 Nov 15;96(9):791-9. doi: 10.1097/TP.0b013e3182a3a52b. Transplantation. 2013. PMID: 23958924
-
Mannose-binding lectin (MBL) and vascular complications in diabetes.Horm Metab Res. 2005 Apr;37 Suppl 1:95-8. doi: 10.1055/s-2005-861372. Horm Metab Res. 2005. PMID: 15918118 Review.
-
Emerging role of the mannose-binding lectin-dependent pathway of complement activation in clinical organ transplantation.Curr Opin Organ Transplant. 2011 Feb;16(1):28-33. doi: 10.1097/MOT.0b013e3283425509. Curr Opin Organ Transplant. 2011. PMID: 21157341 Review.
Cited by
-
New concepts of complement in allorecognition and graft rejection.Cell Immunol. 2007 Jul;248(1):18-30. doi: 10.1016/j.cellimm.2007.04.009. Epub 2007 Oct 24. Cell Immunol. 2007. PMID: 17950717 Free PMC article. Review.
-
The role of the complement system in acute kidney injury.Semin Nephrol. 2013 Nov;33(6):543-56. doi: 10.1016/j.semnephrol.2013.08.005. Semin Nephrol. 2013. PMID: 24161039 Free PMC article. Review.
-
Poly(ADP-ribosyl)ation of mannose-binding lectin out of human kidney cells.Mol Cell Biochem. 2011 Jun;352(1-2):231-8. doi: 10.1007/s11010-011-0758-9. Epub 2011 Mar 6. Mol Cell Biochem. 2011. PMID: 21380727
-
Recent advances into the role of pattern recognition receptors in transplantation.Cell Immunol. 2020 May;351:104088. doi: 10.1016/j.cellimm.2020.104088. Epub 2020 Mar 7. Cell Immunol. 2020. PMID: 32183988 Free PMC article. Review.
-
ON VASCULAR STENOSIS, RESTENOSIS AND MANNOSE BINDING LECTIN.Arq Bras Cir Dig. 2016 Mar;29(1):57-9. doi: 10.1590/0102-6720201600010015. Arq Bras Cir Dig. 2016. PMID: 27120743 Free PMC article. Review.
References
-
- Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality: a cohort analysis. JAMA. 1996;275:1489–1494. - PubMed
-
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. - PubMed
-
- Nicholson ML, Metcalfe MS, White SA, Waller JR, Doughman TM, Horsburgh T, Feehally J, Carr SJ, Veitch PS. A comparison of the results of renal transplantation from non-heart-beating, conventional cadaveric, and living donors. Kidney Int. 2000;58:2585–2591. - PubMed
-
- Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie DP, Taylor SM. A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int. 2003;63:134–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

