Human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T cells that proliferate in vitro detected in samples from most viremic subjects and inversely associated with plasma HIV-1 levels
- PMID: 15507650
- PMCID: PMC525069
- DOI: 10.1128/JVI.78.22.12638-12646.2004
Human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T cells that proliferate in vitro detected in samples from most viremic subjects and inversely associated with plasma HIV-1 levels
Abstract
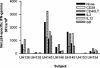
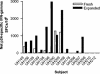
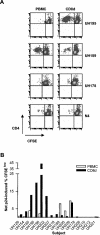
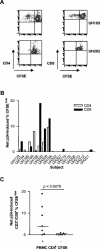
Diminished in vitro proliferation of human immunodeficiency virus type 1 (HIV-1)-specific CD4+T cells has been associated with HIV-1 viremia and declining CD4+ T-cell counts during chronic infection. To better understand this phenomenon, we examined whether HIV-1 Gag p24 antigen-induced CD4+ T-cell proliferation might recover in vitro in a group of subjects with chronic HIV-1 viremia and no history of antiretroviral therapy (ART). We found that depletion of CD8+ cells from peripheral blood mononuclear cells (PBMC) before antigen stimulation was associated with a 6.5-fold increase in the median p24-induced CD4+ T-cell proliferative response and a 57% increase in the number of subjects with positive responses. These p24-induced CD4+ T-cell proliferative responses from CD8-depleted PBMC were associated with expansion of the numbers of p24-specific, gamma interferon (IFN-gamma)-producing CD4+ T cells. Among the 20 viremic, treatment-naive subjects studied, the only 5 subjects lacking proliferation-competent, p24-specific CD4+ T-cell responses from CD8-depleted PBMC showed plasma HIV-1 RNA levels > 100,000 copies/ml. Furthermore, both the magnitude of p24-induced CD4+ T-cell proliferative responses from CD8-depleted PBMC and the frequency of p24-specific, IFN-gamma-producing CD4+ T cells expanded from CD8-depleted PBMC were associated inversely with plasma HIV-1 RNA levels. Therefore, proliferation-competent, HIV-1-specific CD4+ T cells that might help control HIV-1 disease may persist during chronic, progressive HIV-1 disease except at very high levels of in vivo HIV-1 replication.
Figures


Similar articles
-
HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity.J Exp Med. 2003 Dec 15;198(12):1909-22. doi: 10.1084/jem.20031598. J Exp Med. 2003. PMID: 14676302 Free PMC article.
-
Reconstitution of virus-specific CD4 proliferative responses in pediatric HIV-1 infection.J Immunol. 2003 Dec 15;171(12):6968-75. doi: 10.4049/jimmunol.171.12.6968. J Immunol. 2003. PMID: 14662905
-
Polyfunctional CD4(+) T cell responses in HIV-1-infected viral controllers compared with those in healthy recipients of an adjuvanted polyprotein HIV-1 vaccine.Vaccine. 2013 Aug 12;31(36):3739-46. doi: 10.1016/j.vaccine.2013.05.021. Epub 2013 May 21. Vaccine. 2013. PMID: 23707169 Clinical Trial.
-
Immune stimulation and HIV-1 viral replication.J Leukoc Biol. 1997 Jul;62(1):67-71. doi: 10.1002/jlb.62.1.67. J Leukoc Biol. 1997. PMID: 9225995 Review.
-
Viral RNA and p24 antigen as markers of HIV disease and antiretroviral treatment success.Int Arch Allergy Immunol. 2003 Nov;132(3):196-209. doi: 10.1159/000074552. Int Arch Allergy Immunol. 2003. PMID: 14646380 Review.
Cited by
-
Design and preclinical development of a recombinant protein and DNA plasmid mixed format vaccine to deliver HIV-derived T-lymphocyte epitopes.Vaccine. 2009 Nov 23;27(50):7087-95. doi: 10.1016/j.vaccine.2009.09.059. Epub 2009 Sep 26. Vaccine. 2009. PMID: 19786132 Free PMC article.
-
CD4+ T cell targeting of human immunodeficiency virus type 1 (HIV-1) peptide sequences present in vivo during chronic, progressive HIV-1 disease.Virology. 2007 Apr 25;361(1):34-44. doi: 10.1016/j.virol.2006.10.040. Epub 2006 Dec 13. Virology. 2007. PMID: 17169395 Free PMC article.
-
IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells.Blood. 2009 Jul 9;114(2):346-56. doi: 10.1182/blood-2008-12-191296. Epub 2009 Apr 13. Blood. 2009. PMID: 19365081 Free PMC article.
-
Factors associated with viral rebound in HIV-1-infected individuals enrolled in a therapeutic HIV-1 gag vaccine trial.J Infect Dis. 2011 Apr 1;203(7):976-83. doi: 10.1093/infdis/jiq143. J Infect Dis. 2011. PMID: 21402549 Free PMC article. Clinical Trial.
-
Relevance of studying T cell responses in SIV-infected rhesus macaques.Trends Microbiol. 2008 Dec;16(12):605-11. doi: 10.1016/j.tim.2008.08.010. Epub 2008 Oct 27. Trends Microbiol. 2008. PMID: 18964016 Free PMC article. Review.
References
-
- Al-Harthi, L., J. Siegel, J. Spritzler, J. Pottage, M. Agnoli, and A. Landay. 2000. Maximum suppression of HIV replication leads to the restoration of HIV-specific responses in early HIV disease. AIDS 14:761-770. - PubMed
-
- Angel, J. B., K. G. Parato, A. Kumar, S. Kravcik, A. D. Badley, C. Fex, D. Ashby, E. Sun, and D. W. Cameron. 2001. Progressive human immunodeficiency virus-specific immune recovery with prolonged viral suppression. J. Infect. Dis. 183:546-554. - PubMed
-
- Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. - PMC - PubMed
-
- Boaz, M. J., A. Waters, S. Murad, P. J. Easterbrook, and A. Vyakarnam. 2002. Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J. Immunol. 169:6376-6385. - PubMed
-
- Boritz, E., B. E. Palmer, B. Livingston, A. Sette, and C. C. Wilson. 2003. Diverse repertoire of HIV-1 p24-specific, IFN-gamma-producing CD4+ T cell clones following immune reconstitution on highly active antiretroviral therapy. J. Immunol. 170:1106-1116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

