Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo
- PMID: 15504819
- PMCID: PMC2211859
- DOI: 10.1084/jem.20040327
Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo
Abstract
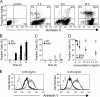
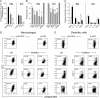
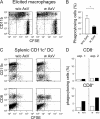
Strategies to enhance the immunogenicity of tumors are urgently needed. Although vaccination with irradiated dying lymphoma cells recruits a tumor-specific immune response, its efficiency as immunogen is poor. Annexin V (AxV) binds with high affinity to phosphatidylserine on the surface of apoptotic and necrotic cells and thereby impairs their uptake by macrophages. Here, we report that AxV preferentially targets irradiated lymphoma cells to CD8+ dendritic cells for in vivo clearance, elicits the release of proinflammatory cytokines and dramatically enhances the protection elicited against the tumor. The response was endowed with both memory, because protected animals rejected living lymphoma cells after 72 d, and specificity, because vaccinated animals failed to reject unrelated neoplasms. Finally, AxV-coupled irradiated cells induced the regression of growing tumors. These data indicate that endogenous adjuvants that bind to dying tumor cells can be exploited to target tumors for immune rejection.
Figures

Similar articles
-
The influence on the immunomodulatory effects of dying and dead cells of Annexin V.J Leukoc Biol. 2007 Jan;81(1):6-14. doi: 10.1189/jlb.0306166. Epub 2006 Sep 27. J Leukoc Biol. 2007. PMID: 17005907
-
AnnexinA5 renders dead tumor cells immunogenic--implications for multimodal cancer therapies.J Immunotoxicol. 2009 Dec;6(4):209-16. doi: 10.3109/15476910903204058. J Immunotoxicol. 2009. PMID: 19908939
-
Exposure of anionic phospholipids serves as anti-inflammatory and immunosuppressive signal--implications for antiphospholipid syndrome and systemic lupus erythematosus.Immunobiology. 2003;207(1):73-81. doi: 10.1078/0171-2985-00217. Immunobiology. 2003. PMID: 12638907
-
Modulation of the immune system by dying cells and the phosphatidylserine-ligand annexin A5.Autoimmunity. 2007 Jun;40(4):254-9. doi: 10.1080/08916930701357331. Autoimmunity. 2007. PMID: 17516206 Review.
-
The role of annexin A5 in the modulation of the immune response against dying and dead cells.Curr Med Chem. 2007;14(3):271-7. doi: 10.2174/092986707779941131. Curr Med Chem. 2007. PMID: 17305532 Review.
Cited by
-
Antibody-Mediated Phosphatidylserine Blockade Enhances the Antitumor Responses to CTLA-4 and PD-1 Antibodies in Melanoma.Cancer Immunol Res. 2016 Jun;4(6):531-40. doi: 10.1158/2326-6066.CIR-15-0250. Epub 2016 Apr 4. Cancer Immunol Res. 2016. PMID: 27045021 Free PMC article.
-
Autophagy proteins regulate cell engulfment mechanisms that participate in cancer.Semin Cancer Biol. 2013 Oct;23(5):329-36. doi: 10.1016/j.semcancer.2013.05.004. Epub 2013 May 30. Semin Cancer Biol. 2013. PMID: 23726896 Free PMC article. Review.
-
Phosphatidylserine-targeting antibodies augment the anti-tumorigenic activity of anti-PD-1 therapy by enhancing immune activation and downregulating pro-oncogenic factors induced by T-cell checkpoint inhibition in murine triple-negative breast cancers.Breast Cancer Res. 2016 May 11;18(1):50. doi: 10.1186/s13058-016-0708-2. Breast Cancer Res. 2016. PMID: 27169467 Free PMC article.
-
Interface between Resolvins and Efferocytosis in Health and Disease.Cell Biochem Biophys. 2024 Mar;82(1):53-65. doi: 10.1007/s12013-023-01187-4. Epub 2023 Oct 4. Cell Biochem Biophys. 2024. PMID: 37794303 Review.
-
Apoptotic cell clearance: basic biology and therapeutic potential.Nat Rev Immunol. 2014 Mar;14(3):166-80. doi: 10.1038/nri3607. Epub 2014 Jan 31. Nat Rev Immunol. 2014. PMID: 24481336 Free PMC article. Review.
References
-
- Savill, J., C. Gregory, and C. Haslett. 2003. Cell biology. Eat me or die. Science. 302:1516–1517. - PubMed
-
- deCathelineau, A.M., and P.M. Henson. 2003. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem. 39:105–117. - PubMed
-
- Albert, M.L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392:86–89. - PubMed
-
- Larsson, M., J.F. Fonteneaou, and N. Bhardwaj. 2001. Dendritic cells resurrect antigens from dead cells. Trends Immunol. 22:142–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

