NEDD4-2 (neural precursor cell expressed, developmentally down-regulated 4-2) negatively regulates TGF-beta (transforming growth factor-beta) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-beta type I receptor
- PMID: 15496141
- PMCID: PMC1134864
- DOI: 10.1042/BJ20040738
NEDD4-2 (neural precursor cell expressed, developmentally down-regulated 4-2) negatively regulates TGF-beta (transforming growth factor-beta) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-beta type I receptor
Abstract
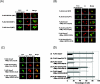
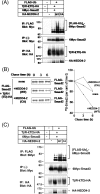
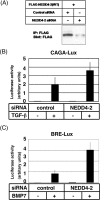
Inhibitory Smad, Smad7, is a potent inhibitor of TGF-beta (transforming growth factor-beta) superfamily signalling. By binding to activated type I receptors, it prevents the activation of R-Smads (receptor-regulated Smads). To identify new components of the Smad pathway, we performed yeast two-hybrid screening using Smad7 as bait, and identified NEDD4-2 (neural precursor cell expressed, developmentally down-regulated 4-2) as a direct binding partner of Smad7. NEDD4-2 is structurally similar to Smurfs (Smad ubiquitin regulatory factors) 1 and 2, which were identified previously as E3 ubiquitin ligases for R-Smads and TGF-beta superfamily receptors. NEDD4-2 functions like Smurfs 1 and 2 in that it associates with TGF-beta type I receptor via Smad7, and induces its ubiquitin-dependent degradation. Moreover, NEDD4-2 bound to TGF-beta-specific R-Smads, Smads 2 and 3, in a ligand-dependent manner, and induced degradation of Smad2, but not Smad3. However, in contrast with Smurf2, NEDD4-2 failed to induce ubiquitination of SnoN (Ski-related novel protein N), although NEDD4-2 bound to SnoN via Smad2 more strongly than Smurf2. We showed further that overexpressed NEDD4-2 prevents transcriptional activity induced by TGF-beta and BMP, whereas silencing of the NEDD4-2 gene by siRNA (small interfering RNA) resulted in enhancement of the responsiveness to TGF-beta superfamily cytokines. These data suggest that NEDD4-2 is a member of the Smurf-like C2-WW-HECT (WW is Trp-Trp and HECT is homologous to the E6-accessory protein) type E3 ubiquitin ligases, which negatively regulate TGF-beta superfamily signalling through similar, but not identical, mechanisms to those used by Smurfs.
Figures





Similar articles
-
Negative regulation of transforming growth factor-beta (TGF-beta) signaling by WW domain-containing protein 1 (WWP1).Oncogene. 2004 Sep 9;23(41):6914-23. doi: 10.1038/sj.onc.1207885. Oncogene. 2004. PMID: 15221015
-
Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases.J Biol Chem. 2005 Jun 10;280(23):22115-23. doi: 10.1074/jbc.M414027200. Epub 2005 Apr 6. J Biol Chem. 2005. PMID: 15817471
-
The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling.J Cell Biol. 2001 Dec 10;155(6):1017-27. doi: 10.1083/jcb.200106023. Epub 2001 Dec 10. J Cell Biol. 2001. PMID: 11739411 Free PMC article.
-
Regulation of transforming growth factor-beta signaling.Mol Cell Biol Res Commun. 2001 Nov;4(6):321-30. doi: 10.1006/mcbr.2001.0301. Mol Cell Biol Res Commun. 2001. PMID: 11703090 Review.
-
Regulation of TGF-beta family signaling by E3 ubiquitin ligases.Cancer Sci. 2008 Nov;99(11):2107-12. doi: 10.1111/j.1349-7006.2008.00925.x. Epub 2008 Sep 18. Cancer Sci. 2008. PMID: 18808420 Free PMC article. Review.
Cited by
-
The E3 Ligases in Cervical Cancer and Endometrial Cancer.Cancers (Basel). 2022 Oct 30;14(21):5354. doi: 10.3390/cancers14215354. Cancers (Basel). 2022. PMID: 36358773 Free PMC article. Review.
-
Differential regulation of Smad3 and of the type II transforming growth factor-β receptor in mitosis: implications for signaling.PLoS One. 2012;7(8):e43459. doi: 10.1371/journal.pone.0043459. Epub 2012 Aug 22. PLoS One. 2012. PMID: 22927969 Free PMC article.
-
Eps15R is required for bone morphogenetic protein signalling and differentially compartmentalizes with Smad proteins.Open Biol. 2012 Apr;2(4):120060. doi: 10.1098/rsob.120060. Open Biol. 2012. PMID: 22724065 Free PMC article.
-
Key role for ubiquitin protein modification in TGFβ signal transduction.Ups J Med Sci. 2012 May;117(2):153-65. doi: 10.3109/03009734.2012.654858. Epub 2012 Feb 15. Ups J Med Sci. 2012. PMID: 22335355 Free PMC article. Review.
-
The correlation between NEDD4L and HIF-1α levels as a gastric cancer prognostic marker.Int J Med Sci. 2019 Oct 21;16(11):1517-1524. doi: 10.7150/ijms.34646. eCollection 2019. Int J Med Sci. 2019. PMID: 31673244 Free PMC article.
References
-
- Roberts A. B., Sporn M. B. The transforming growth factor-βs. In: Sporn M. B., Roberts A. B., editors. Peptide Growth Factors and Their Receptors, Part I. Heidelberg: Springer-Verlag; 1990. pp. 419–472.
-
- Heldin C.-H., Miyazono K., ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature (London) 1997;390:465–471. - PubMed
-
- Massagué J. TGF-β signal transduction. Annu. Rev. Biochem. 1998;67:753–791. - PubMed
-
- Derynck R., Zhang Y., Feng X.-H. Smads: transcriptional activators of TGF-β responses. Cell. 1998;95:737–740. - PubMed
-
- Attisano L., Wrana J. L. Smads as transcriptional co-modulators. Curr. Opin. Cell Biol. 2000;12:235–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

