Fas-associated protein with death domain (FADD)-independent recruitment of c-FLIPL to death receptor 5
- PMID: 15485835
- PMCID: PMC2981793
- DOI: 10.1074/jbc.M401056200
Fas-associated protein with death domain (FADD)-independent recruitment of c-FLIPL to death receptor 5
Abstract
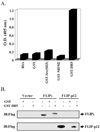
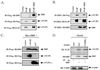
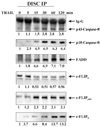
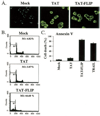
Here we show a novel mechanism by which FLICE-like inhibitory protein (c-FLIP) regulates apoptosis induced by tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and one of its receptors, DR5. c-FLIP is a critical regulator of the TNF family of cytokine receptor signaling. c-FLIP has been postulated to prevent formation of the competent death-inducing signaling complex (DISC) in a ligand-dependent manner, through its interaction with FADD and/or caspase-8. In order to identify regulators of TRAIL function, we used the intracellular death domain (DD) of DR5 as a target to screen a phage-displayed combinatorial peptide library. The DD of DR5 selected from the library a peptide that showed sequence similarity to a stretch of amino acids in the C terminus of c-FLIP(L). The phage-displayed peptide selectively interacted with the DD of DR5 in in vitro binding assays. Similarly, full-length c-FLIP (c-FLIP(L)) and the C-terminal p12 domain of c-FLIP interacted with DR5 both in in vitro pull-down assays and in mammalian cells. This interaction was independent of TRAIL. To the contrary, TRAIL treatment released c-FLIP(L) from DR5, permitting the recruitment of FADD to the active DR5 signaling complex. By employing FADD-deficient Jurkat cells, we demonstrate that DR5 and c-FLIP(L) interact in a FADD-independent manner. Moreover, we show that a cellular membrane permeable version of the peptide corresponding to the DR5 binding domain of c-FLIP induces apoptosis in mammalian cells. Taken together, these findings indicate that c-FLIP interacts with the DD of DR5, thus preventing death (L)signaling by DR5 prior to the formation of an active DISC. Because TRAIL and DR5 are ubiquitously expressed, the interaction of c-FLIP(L) and DR5 indicates a mechanism by which tumor selective apoptosis can be achieved through protecting normal cells from undergoing death receptor-induced apoptosis.
Figures

Similar articles
-
c-FLIP, a master anti-apoptotic regulator.Exp Oncol. 2012 Oct;34(3):176-84. Exp Oncol. 2012. PMID: 23070002 Free PMC article. Review.
-
Fas-associated death domain protein and caspase-8 are not recruited to the tumor necrosis factor receptor 1 signaling complex during tumor necrosis factor-induced apoptosis.J Biol Chem. 2003 Jul 11;278(28):25534-41. doi: 10.1074/jbc.M303399200. Epub 2003 Apr 29. J Biol Chem. 2003. PMID: 12721308
-
Fas-associated death domain protein (FADD) and caspase-8 mediate up-regulation of c-Fos by Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) via a FLICE inhibitory protein (FLIP)-regulated pathway.J Biol Chem. 2001 Aug 31;276(35):32585-90. doi: 10.1074/jbc.M100444200. Epub 2001 May 30. J Biol Chem. 2001. PMID: 11384965
-
Human astrocytes are resistant to Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis.J Neurosci. 2006 Mar 22;26(12):3299-308. doi: 10.1523/JNEUROSCI.5572-05.2006. J Neurosci. 2006. PMID: 16554480 Free PMC article.
-
TRAIL-induced signalling and apoptosis.Toxicol Lett. 2003 Apr 4;139(2-3):89-97. doi: 10.1016/s0378-4274(02)00422-8. Toxicol Lett. 2003. PMID: 12628743 Review.
Cited by
-
TRAIL recombinant adenovirus triggers robust apoptosis in multidrug-resistant HL-60/Vinc cells preferentially through death receptor DR5.Hum Gene Ther. 2008 Jul;19(7):731-43. doi: 10.1089/hum.2008.001. Hum Gene Ther. 2008. PMID: 18476767 Free PMC article.
-
c-FLIP, a master anti-apoptotic regulator.Exp Oncol. 2012 Oct;34(3):176-84. Exp Oncol. 2012. PMID: 23070002 Free PMC article. Review.
-
Roles of c-FLIP in Apoptosis, Necroptosis, and Autophagy.J Carcinog Mutagen. 2013;Suppl 6:003. doi: 10.4172/2157-2518.S6-003. J Carcinog Mutagen. 2013. PMID: 25379355 Free PMC article.
-
Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2.Mol Cell Biol. 2006 Oct;26(19):7046-55. doi: 10.1128/MCB.00520-06. Mol Cell Biol. 2006. PMID: 16980609 Free PMC article.
-
Cellular FLICE-like inhibitory protein (C-FLIP): a novel target for cancer therapy.Curr Cancer Drug Targets. 2008 Feb;8(1):37-46. doi: 10.2174/156800908783497087. Curr Cancer Drug Targets. 2008. PMID: 18288942 Free PMC article. Review.
References
-
- Abe K, Kurakin A, Mohseni-Maybodi M, Kay B, Khosravi-Far R. Ann. N. Y. Acad. Sci. 2000;926:52–63. - PubMed
-
- Ashkenazi A, Dixit VM. Curr. Opin. Cell Biol. 1999;11:255–260. - PubMed
-
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. J. Biol. Chem. 1996;271:12687–12690. - PubMed
-
- Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Eur. J. Biochem. 1998;254:439–459. - PubMed
-
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Immunity. 1995;3:673–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

