Ral and phospholipase D2-dependent pathway for constitutive metabotropic glutamate receptor endocytosis
- PMID: 15470141
- PMCID: PMC6729950
- DOI: 10.1523/JNEUROSCI.3155-04.2004
Ral and phospholipase D2-dependent pathway for constitutive metabotropic glutamate receptor endocytosis
Abstract
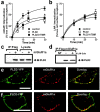
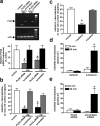
G-protein-coupled receptors play a central role in the regulation of neuronal cell communication. Class 1 metabotropic glutamate receptors (mGluRs) mGluR1a and mGluR5a, which are coupled with the hydrolysis of phosphoinositides, are essential for modulating excitatory neurotransmission at glutamatergic synapses. These receptors are constitutively internalized in heterologous cell cultures, neuronal cultures, and intact neuronal tissues. We show here that the small GTP-binding protein Ral, its guanine nucleotide exchange factor RalGDS (Ral GDP dissociation stimulator), and phospholipase D2 (PLD2) are constitutively associated with class 1 mGluRs and regulate constitutive mGluR endocytosis. Moreover, both Ral and PLD2 are colocalized with mGluRs in endocytic vesicles in both human embryonic kidney 293 (HEK 293) cells and neurons. Ral and PLD2 activity is required for the internalization of class 1 mGluRs but is not required for the internalization of the beta2-adrenergic receptor. Constitutive class 1 mGluR internalization is not dependent on the downstream Ral effector proteins Ral-binding protein 1 and PLD1 or either ADP-ribosylation factors ARF1 or ARF6. The treatment of HEK 293 cells and neurons with small interfering RNA both downregulates PLD2 expression and blocks mGluR1a and mGluR5a endocytosis. The constitutive internalization of mGluR1a and mGluR5a is also attenuated by the treatment of cells with 1-butanol to prevent PLD2-mediated phosphatidic acid formation. We propose that the formation of a mGluR-scaffolded RalGDS/Ral/PLD2 protein complex provides a novel alternative mechanism to beta-arrestins for the constitutive endocytosis of class 1 mGluRs.
Figures




Similar articles
-
Agonist-stimulated and tonic internalization of metabotropic glutamate receptor 1a in human embryonic kidney 293 cells: agonist-stimulated endocytosis is beta-arrestin1 isoform-specific.Mol Pharmacol. 2001 Dec;60(6):1243-53. doi: 10.1124/mol.60.6.1243. Mol Pharmacol. 2001. PMID: 11723231
-
Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis.Pharmacol Ther. 2006 Jul;111(1):260-71. doi: 10.1016/j.pharmthera.2005.01.008. Epub 2006 Mar 6. Pharmacol Ther. 2006. PMID: 16574233 Review.
-
Calcineurin inhibitor protein (CAIN) attenuates Group I metabotropic glutamate receptor endocytosis and signaling.J Biol Chem. 2009 Oct 16;284(42):28986-94. doi: 10.1074/jbc.M109.050872. Epub 2009 Aug 28. J Biol Chem. 2009. PMID: 19717561 Free PMC article.
-
BRAG2a Mediates mGluR-Dependent AMPA Receptor Internalization at Excitatory Postsynapses through the Interaction with PSD-95 and Endophilin 3.J Neurosci. 2020 May 27;40(22):4277-4296. doi: 10.1523/JNEUROSCI.1645-19.2020. Epub 2020 Apr 27. J Neurosci. 2020. PMID: 32341099 Free PMC article.
-
Mechanisms of metabotropic glutamate receptor desensitization: role in the patterning of effector enzyme activation.Neurochem Int. 2002 Nov;41(5):319-26. doi: 10.1016/s0197-0186(02)00073-6. Neurochem Int. 2002. PMID: 12176073 Review.
Cited by
-
RalGTPases contribute to Schwann cell repair after nerve injury via regulation of process formation.J Cell Biol. 2019 Jul 1;218(7):2370-2387. doi: 10.1083/jcb.201811002. Epub 2019 Jun 14. J Cell Biol. 2019. PMID: 31201266 Free PMC article.
-
The Lipase Activity of Phospholipase D2 is Responsible for Nigral Neurodegeneration in a Rat Model of Parkinson's Disease.Neuroscience. 2018 May 1;377:174-183. doi: 10.1016/j.neuroscience.2018.02.047. Epub 2018 Mar 9. Neuroscience. 2018. PMID: 29526688 Free PMC article.
-
Diacylglycerol, phosphatidic acid, and their metabolic enzymes in synaptic vesicle recycling.Adv Biol Regul. 2015 Jan;57:147-52. doi: 10.1016/j.jbior.2014.09.010. Epub 2014 Sep 28. Adv Biol Regul. 2015. PMID: 25446883 Free PMC article. Review.
-
Rab8 modulates metabotropic glutamate receptor subtype 1 intracellular trafficking and signaling in a protein kinase C-dependent manner.J Neurosci. 2012 Nov 21;32(47):16933-42a. doi: 10.1523/JNEUROSCI.0625-12.2012. J Neurosci. 2012. PMID: 23175844 Free PMC article.
-
Phosphatidic acid induces ligand-independent epidermal growth factor receptor endocytic traffic through PDE4 activation.Mol Biol Cell. 2010 Aug 15;21(16):2916-29. doi: 10.1091/mbc.E10-02-0167. Epub 2010 Jun 16. Mol Biol Cell. 2010. PMID: 20554760 Free PMC article.
References
-
- Arneson LS, Kunz J, Anderson RA, Traub LM (1999) Coupled inositide phosphorylation and phospholipase D activation initiates clathrin-coat assembly on lysosomes. J Biol Chem 274: 17794-17805. - PubMed
-
- Bhattacharya M, Anborgh PH, Babwah AV, Dale LB, Dobransky T, Benovic JL, Feldman RD, Verdi JM, Rylett RJ, Ferguson SSG (2002) β-Arrestins regulate a Ral-GDS Ral effector pathway that mediates cytoskeletal reorganization. Nat Cell Biol 4: 547-555. - PubMed
-
- Bhowmick N, Narayan P, Puett D (1998) The endothelin subtype A receptor undergoes agonist- and antagonist-mediated internalization in the absence of signaling. Endocrinology 139: 3185-3192. - PubMed
-
- Cao TT, Mays RW, von Zastrow M (1998) Regulated endocytosis of G-protein-coupled receptors by a biochemically and functionally distinct subpopulation of clathrin-coated pits. J Biol Chem 273: 24592-24602. - PubMed
-
- Carroll RC, Zukin RS (2002) NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci 25: 571-577. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
