Anti-Vpr activity of a yeast chaperone protein
- PMID: 15452222
- PMCID: PMC521794
- DOI: 10.1128/JVI.78.20.11016-11029.2004
Anti-Vpr activity of a yeast chaperone protein
Abstract
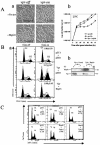
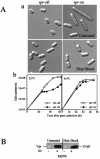
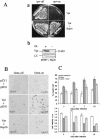
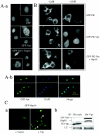
Human immunodeficiency virus type 1 (HIV-1) viral protein R (Vpr) exerts multiple effects on viral and host cellular activities during viral infection, including nuclear transport of the proviral integration complex, induction of cell cycle G(2) arrest, and cell death. In this report, we show that a fission yeast chaperone protein Hsp16 inhibits HIV-1 by suppressing these Vpr activities. This protein was identified through three independent genome-wide screens for multicopy suppressors of each of the three Vpr activities. Consistent with the properties of a heat shock protein, heat shock-induced elevation or overproduction of Hsp16 suppressed Vpr activities through direct protein-protein interaction. Even though Hsp16 shows a stronger suppressive effect on Vpr in fission yeast than in mammalian cells, similar effects were also observed in human cells when fission yeast hsp16 was expressed either in vpr-expressing cells or during HIV-1 infection, indicating a possible highly conserved Vpr suppressing activity. Furthermore, stable expression of hsp16 prior to HIV-1 infection inhibits viral replication in a Vpr-dependent manner. Together, these data suggest that Hsp16 inhibits HIV-1 by suppressing Vpr-specific activities. This finding could potentially provide a new approach to studying the contribution of Vpr to viral pathogenesis and to reducing Vpr-mediated detrimental effects in HIV-infected patients.
Figures

Similar articles
-
Antagonistic interaction of HIV-1 Vpr with Hsf-mediated cellular heat shock response and Hsp16 in fission yeast (Schizosaccharomyces pombe).Retrovirology. 2007 Mar 7;4:16. doi: 10.1186/1742-4690-4-16. Retrovirology. 2007. PMID: 17341318 Free PMC article.
-
Heat-shock proteins reverse the G2 arrest caused by HIV-1 viral protein R.DNA Cell Biol. 2004 Apr;23(4):223-5. doi: 10.1089/104454904773819806. DNA Cell Biol. 2004. PMID: 15142379
-
Suppressive effect of elongation factor 2 on apoptosis induced by HIV-1 viral protein R.Apoptosis. 2006 Mar;11(3):377-88. doi: 10.1007/s10495-006-4030-9. Apoptosis. 2006. PMID: 16520893
-
Yeast perspectives on HIV-1 VPR.Front Biosci. 2000 Dec 1;5:D905-16. doi: 10.2741/zhao. Front Biosci. 2000. PMID: 11102318 Review.
-
Partner molecules of accessory protein Vpr of the human immunodeficiency virus type 1.DNA Cell Biol. 2004 Apr;23(4):193-205. doi: 10.1089/104454904773819789. DNA Cell Biol. 2004. PMID: 15142377 Review.
Cited by
-
HIV-1 viral protein R (Vpr) and its interactions with host cell.Curr HIV Res. 2009 Mar;7(2):178-83. doi: 10.2174/157016209787581436. Curr HIV Res. 2009. PMID: 19275587 Free PMC article. Review.
-
The dose-dependent H2O2 stress response promotes increased survival for Schizosaccharomyces pombe cells expressing HIV-1 Vpr.Folia Microbiol (Praha). 2006;51(5):406-12. doi: 10.1007/BF02931584. Folia Microbiol (Praha). 2006. PMID: 17176760
-
Cell cycle G2/M arrest through an S phase-dependent mechanism by HIV-1 viral protein R.Retrovirology. 2010 Jul 7;7:59. doi: 10.1186/1742-4690-7-59. Retrovirology. 2010. PMID: 20609246 Free PMC article.
-
HIV-1 Protease in the Fission Yeast Schizosaccharomyces pombe.PLoS One. 2016 Mar 16;11(3):e0151286. doi: 10.1371/journal.pone.0151286. eCollection 2016. PLoS One. 2016. PMID: 26982200 Free PMC article.
-
Antagonistic interaction of HIV-1 Vpr with Hsf-mediated cellular heat shock response and Hsp16 in fission yeast (Schizosaccharomyces pombe).Retrovirology. 2007 Mar 7;4:16. doi: 10.1186/1742-4690-4-16. Retrovirology. 2007. PMID: 17341318 Free PMC article.
References
-
- Agostini, I., S. Popov, T. Hao, J. H. Li, L. Dubrovsky, O. Chaika, N. Chaika, R. Lewis, and M. Bukrinsky. 2002. Phosphorylation of Vpr regulates HIV type 1 nuclear import and macrophage infection. PG-283-8. AIDS Res. Hum. Retrovir. 18:283-288. - PubMed
-
- Agostini, I., S. Popov, J. Li, L. Dubrovsky, T. Hao, and M. Bukrinsky. 2000. Heat-shock protein 70 can replace viral protein R of HIV-1 during nuclear import of the viral preintegration complex. Exp. Cell Res. 259:398-403. - PubMed
-
- Ayyavoo, V., S. Mahalingam, Y. Rafaeli, S. Kudchodkar, D. Chang, T. Nagashunmugam, W. V. Williams, and D. B. Weiner. 1997. HIV-1 viral protein R (Vpr) regulates viral replication and cellular proliferation in T cells and monocytoid cells in vitro. J. Leukoc. Biol. 62:93-99. - PubMed
-
- Barre-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vezinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868-871. - PubMed
-
- Brenner, B. G., Y. Tao, E. Pearson, I. Remer, and M. A. Wainberg. 1995. Altered constitutive and stress-regulated heat shock protein 27 expression in HIV type 1-infected cell lines. AIDS Res. Hum. Retrovir. 11:713-717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

