Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts
- PMID: 15385625
- PMCID: PMC532013
- DOI: 10.1091/mbc.e04-05-0385
Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts
Abstract
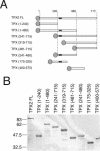
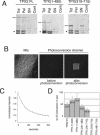
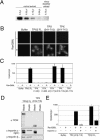
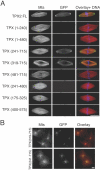
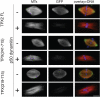
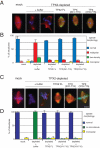
TPX2 has multiple functions during mitosis, including microtubule nucleation around the chromosomes and the targeting of Xklp2 and Aurora A to the spindle. We have performed a detailed domain functional analysis of TPX2 and found that a large N-terminal domain containing the Aurora A binding peptide interacts directly with and nucleates microtubules in pure tubulin solutions. However, it cannot substitute the endogenous TPX2 to support microtubule nucleation in response to Ran guanosine triphosphate (GTP) and spindle assembly in egg extracts. By contrast, a large C-terminal domain of TPX2 that does not bind directly to pure microtubules and does not bind Aurora A kinase rescues microtubule nucleation in response to RanGTP and spindle assembly in TPX2-depleted extract. These and previous results suggest that under physiological conditions, TPX2 is essential for microtubule nucleation around chromatin and functions in a network of other molecules, some of which also are regulated by RanGTP.
Figures



Similar articles
-
Ran modulates spindle assembly by regulating a subset of TPX2 and Kid activities including Aurora A activation.J Cell Sci. 2003 Dec 1;116(Pt 23):4791-8. doi: 10.1242/jcs.00798. J Cell Sci. 2003. PMID: 14600264
-
Microtubule nucleation in mitosis by a RanGTP-dependent protein complex.Curr Biol. 2015 Jan 19;25(2):131-140. doi: 10.1016/j.cub.2014.11.025. Epub 2014 Dec 18. Curr Biol. 2015. PMID: 25532896
-
Cdk11 is a RanGTP-dependent microtubule stabilization factor that regulates spindle assembly rate.J Cell Biol. 2008 Mar 10;180(5):867-75. doi: 10.1083/jcb.200706189. Epub 2008 Mar 3. J Cell Biol. 2008. PMID: 18316407 Free PMC article.
-
Regulation of Aurora-A kinase on the mitotic spindle.Chromosoma. 2003 Dec;112(4):159-63. doi: 10.1007/s00412-003-0265-1. Epub 2003 Nov 21. Chromosoma. 2003. PMID: 14634755 Review.
-
The mechanism of spindle assembly: functions of Ran and its target TPX2.J Cell Biol. 2004 Sep 27;166(7):949-55. doi: 10.1083/jcb.200312112. J Cell Biol. 2004. PMID: 15452138 Free PMC article. Review.
Cited by
-
A FRET biosensor reveals spatiotemporal activation and functions of aurora kinase A in living cells.Nat Commun. 2016 Sep 14;7:12674. doi: 10.1038/ncomms12674. Nat Commun. 2016. PMID: 27624869 Free PMC article.
-
MAP20, a microtubule-associated protein in the secondary cell walls of hybrid aspen, is a target of the cellulose synthesis inhibitor 2,6-dichlorobenzonitrile.Plant Physiol. 2008 Nov;148(3):1283-94. doi: 10.1104/pp.108.121913. Epub 2008 Sep 19. Plant Physiol. 2008. PMID: 18805954 Free PMC article.
-
Control of Aurora-A stability through interaction with TPX2.J Cell Sci. 2011 Jan 1;124(Pt 1):113-22. doi: 10.1242/jcs.075457. Epub 2010 Dec 8. J Cell Sci. 2011. PMID: 21147853 Free PMC article.
-
Structural analysis of the role of TPX2 in branching microtubule nucleation.J Cell Biol. 2017 Apr 3;216(4):983-997. doi: 10.1083/jcb.201607060. Epub 2017 Mar 6. J Cell Biol. 2017. PMID: 28264915 Free PMC article.
-
Sequences in the stalk domain regulate auto-inhibition and ciliary tip localization of the immotile kinesin-4 KIF7.J Cell Sci. 2021 Jul 1;134(13):jcs258464. doi: 10.1242/jcs.258464. Epub 2021 Jul 8. J Cell Sci. 2021. PMID: 34114033 Free PMC article.
References
-
- Bayliss, R., Sardon, T., Vernos, I., and Conti, E. (2003). Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell 12, 851–862. - PubMed
-
- Bornens, M., and Moudjou, M. (1999). Studying the composition and function of centrosomes in vertebrates. Methods Cell Biol. 61, 13–34. - PubMed
-
- Dasso, M. (2001). Running on Ran: nuclear transport and the mitotic spindle. Cell 104, 321–324. - PubMed
-
- Dasso, M. (2002). The Ran GTPase: theme and variations. Curr. Biol. 12, R502–R508. - PubMed
-
- Desai, A., Murray, A., Mitchison, T.J., and Walczak, C.E. (1999). The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61, 385–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

