Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes
- PMID: 15381731
- PMCID: PMC2211971
- DOI: 10.1084/jem.20040254
Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes
Abstract
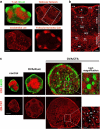
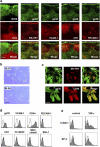
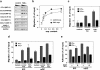
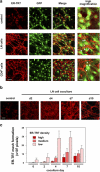
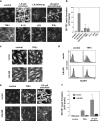
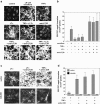
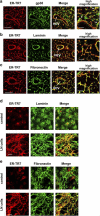
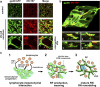
The sophisticated microarchitecture of the lymph node, which is largely supported by a reticular network of fibroblastic reticular cells (FRCs) and extracellular matrix, is essential for immune function. How FRCs form the elaborate network and remodel it in response to lymphocyte activation is not understood. In this work, we established ERTR7(+)gp38(+)VCAM-1(+) FRC lines and examined the production of the ER-TR7 antigen. Multiple chemokines produced by FRCs induced T cell and dendritic cell chemotaxis and adhesion to the FRC surface. FRCs can secrete the ER-TR7 antigen as an extracellular matrix component to make a reticular meshwork in response to contact with lymphocytes. The formation of the meshwork is induced by stimulation with tumor necrosis factor-alpha or lymphotoxin-alpha in combination with agonistic antibody to lymphotoxin-beta receptor in a nuclear factor-kappaB (RelA)-dependent manner. These findings suggest that signals from lymphocytes induce FRCs to form the network that supports the movement and interactions of immune effectors within the lymph node.
Figures
Similar articles
-
A transmembrane chemokine, CXC chemokine ligand 16, expressed by lymph node fibroblastic reticular cells has the potential to regulate T cell migration and adhesion.Int Immunol. 2006 Feb;18(2):301-11. doi: 10.1093/intimm/dxh369. Epub 2006 Jan 12. Int Immunol. 2006. PMID: 16410312
-
A novel reticular stromal structure in lymph node cortex: an immuno-platform for interactions among dendritic cells, T cells and B cells.Int Immunol. 2004 Aug;16(8):1133-42. doi: 10.1093/intimm/dxh113. Epub 2004 Jul 5. Int Immunol. 2004. PMID: 15237106
-
Spleen-derived stromal cells. Adhesion molecules expression and lymphocyte adhesion to reticular cells.Eur J Cell Biol. 1997 Dec;74(4):321-8. Eur J Cell Biol. 1997. PMID: 9438127
-
Communication, construction, and fluid control: lymphoid organ fibroblastic reticular cell and conduit networks.Trends Immunol. 2021 Sep;42(9):782-794. doi: 10.1016/j.it.2021.07.003. Epub 2021 Aug 3. Trends Immunol. 2021. PMID: 34362676 Review.
-
Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic.J Immunol. 1996 Jul 15;157(2):495-9. J Immunol. 1996. PMID: 8752893 Review.
Cited by
-
Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution.Blood. 2012 Aug 30;120(9):1856-67. doi: 10.1182/blood-2012-03-418624. Epub 2012 May 21. Blood. 2012. PMID: 22613799 Free PMC article.
-
A role for LFA-1 in delaying T-lymphocyte egress from lymph nodes.EMBO J. 2013 Mar 20;32(6):829-43. doi: 10.1038/emboj.2013.33. Epub 2013 Feb 26. EMBO J. 2013. PMID: 23443048 Free PMC article.
-
Altered Dermal Fibroblasts in Systemic Sclerosis Display Podoplanin and CD90.Am J Pathol. 2016 Oct;186(10):2650-64. doi: 10.1016/j.ajpath.2016.06.020. Epub 2016 Aug 23. Am J Pathol. 2016. PMID: 27565038 Free PMC article.
-
Tumor-induced stromal reprogramming drives lymph node transformation.Nat Immunol. 2016 Sep;17(9):1118-27. doi: 10.1038/ni.3492. Epub 2016 Jul 11. Nat Immunol. 2016. PMID: 27400148 Free PMC article.
-
Mechanisms of lymphoma-stromal interactions focusing on tumor-associated macrophages, fibroblastic reticular cells, and follicular dendritic cells.J Clin Exp Hematop. 2024 Sep 28;64(3):166-176. doi: 10.3960/jslrt.24034. Epub 2024 Jul 31. J Clin Exp Hematop. 2024. PMID: 39085126 Free PMC article. Review.
References
-
- Zinkernagel, R.M., S. Ehl, P. Aichele, S. Oehen, T. Kündig, and H. Hengartner. 1997. Antigen localization regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol. Revs. 156:199–209. - PubMed
-
- Fu, Y.-X., and D.D. Chaplin. 1999. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17:399–433. - PubMed
-
- Cyster, J.G. 1999. Chemokine and cell migration in secondary lymphoid organs. Science. 286:2098–2102. - PubMed
-
- Young, B., and J.H. Heath. 2000. Wheater's Functional Histology. 4th ed. Harcourt Publishers Limited, London.
-
- Cyster, J.G., K.M. Ansel, K. Reif, E.H. Ekland, P.L. Hyman, H.L. Tang, S.A. Luther, and V.N. Ngo. 2000. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Revs. 176:181–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

