Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex
- PMID: 15371341
- PMCID: PMC517520
- DOI: 10.1101/gad.1214704
Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex
Abstract
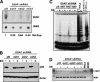
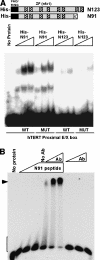
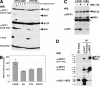
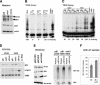
The critical immortalizing activity of the human papillomavirus (HPV) type-16 E6 oncoprotein is to induce expression of hTERT, the catalytic and rate-limiting subunit of telomerase. Additionally, E6 binds to a cellular protein called E6-associated protein (E6-AP) to form an E3 ubiquitin ligase that targets p53 for proteasome-dependent degradation. Although telomerase induction and p53 degradation are separable and distinct functions of E6, binding of E6 to E6-AP strongly correlated with the induction of hTERT. Here, we demonstrate using shRNAs to reduce E6-AP expression that E6-AP is required for E6-mediated telomerase induction. A yeast two-hybrid screen to find new targets of the E6/E6-AP E3 ubiquitin ligase complex identified NFX1. Two isoforms of NFX1 were found: NFX1-123, which coactivated with c-Myc at the hTERT promoter, and NFX1-91, which repressed the hTERT promoter. NFX1-91 was highly ubiquitinated and destabilized in epithelial cells expressing E6. Furthermore, knockdown of NFX1-91 by shRNA resulted in derepression of the endogenous hTERT promoter and elevated levels of telomerase activity. We propose that the induction of telomerase by the HPV-16 E6/E6-AP complex involves targeting of NFX1-91, a newly identified repressor of telomerase, for ubiquitination and degradation.
Figures


Similar articles
-
NFX1-123 and poly(A) binding proteins synergistically augment activation of telomerase in human papillomavirus type 16 E6-expressing cells.J Virol. 2007 Apr;81(8):3786-96. doi: 10.1128/JVI.02007-06. Epub 2007 Jan 31. J Virol. 2007. PMID: 17267499 Free PMC article.
-
Cytoplasmic poly(A) binding proteins regulate telomerase activity and cell growth in human papillomavirus type 16 E6-expressing keratinocytes.J Virol. 2010 Dec;84(24):12934-44. doi: 10.1128/JVI.01377-10. Epub 2010 Oct 13. J Virol. 2010. PMID: 20943973 Free PMC article.
-
Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites.J Virol. 2001 Jun;75(12):5559-66. doi: 10.1128/JVI.75.12.5559-5566.2001. J Virol. 2001. PMID: 11356963 Free PMC article.
-
Activation of telomerase by HPVs.Virus Res. 2017 Mar 2;231:50-55. doi: 10.1016/j.virusres.2016.11.003. Epub 2016 Nov 15. Virus Res. 2017. PMID: 27863966 Review.
-
Telomerase Induction in HPV Infection and Oncogenesis.Viruses. 2017 Jul 10;9(7):180. doi: 10.3390/v9070180. Viruses. 2017. PMID: 28698524 Free PMC article. Review.
Cited by
-
Genes Regulated by HPV 16 E6 and High Expression of NFX1-123 in Cervical Cancers.Onco Targets Ther. 2020 Jun 26;13:6143-6156. doi: 10.2147/OTT.S251926. eCollection 2020. Onco Targets Ther. 2020. PMID: 32617009 Free PMC article.
-
The Molecular Interplay between Human Oncoviruses and Telomerase in Cancer Development.Cancers (Basel). 2022 Oct 26;14(21):5257. doi: 10.3390/cancers14215257. Cancers (Basel). 2022. PMID: 36358677 Free PMC article. Review.
-
Association between hTERT activation by HPV E6 proteins and oncogenic risk.Virology. 2012 Nov 10;433(1):216-9. doi: 10.1016/j.virol.2012.08.006. Epub 2012 Aug 25. Virology. 2012. PMID: 22925336 Free PMC article.
-
HECT E3s and human disease.BMC Biochem. 2007 Nov 22;8 Suppl 1(Suppl 1):S6. doi: 10.1186/1471-2091-8-S1-S6. BMC Biochem. 2007. PMID: 18047743 Free PMC article. Review.
-
Human papillomavirus type 16 E6 and E7 oncoproteins act synergistically to cause head and neck cancer in mice.Virology. 2010 Nov 10;407(1):60-7. doi: 10.1016/j.virol.2010.08.003. Epub 2010 Aug 24. Virology. 2010. PMID: 20797753 Free PMC article.
References
-
- Baege A.C., Berger, A., Schlegel, R., Veldman, T., and Schlegel, R. 2002. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am. J. Pathol. 160: 1251-1257. - PMC - PubMed
-
- Bartz S.R. and Vodicka, M.A. 1997. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods 12: 337-342. - PubMed
-
- Boyer S.N., Wazer, D.E., and Band, V. 1996. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 56: 4620-4624. - PubMed
-
- Chong S.R., Mersha, F.B., Comb, D.G., Scott, M.E., Landry, D., Vence, L.M., Perler, F.B., Benner, J., Kucera, R.B., Hirvonen, C.A., et al. 1997. Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element. Gene 192: 271-281. - PubMed
-
- Cong Y.S. and Bacchetti, S. 2000. Histone deacetylation is involved in the transcriptional repression of hTERT in normal human cells. J. Biol. Chem. 275: 35665-35668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
