Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway
- PMID: 15367621
- PMCID: PMC516396
- DOI: 10.1128/JVI.78.19.10543-10555.2004
Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway
Abstract
The pathway of West Nile flavivirus early internalization events was mapped in detail in this study. Overexpression of dominant-negative mutants of Eps15 strongly inhibits West Nile virus (WNV) internalization, and pharmacological drugs that blocks clathrin also caused a marked reduction in virus entry but not caveola-dependent endocytosis inhibitory agent, filipin. Using immunocryoelectron microscopy, WNV particles were seen within clathrin-coated pits after 2 min postinfection. Double-labeling immunofluorescence assays and immunoelectron microscopy performed with anti-WNV envelope or capsid proteins and cellular markers (EEA1 and LAMP1) revealed the trafficking pathway of internalized virus particles from early endosomes to lysosomes and finally the uncoating of the virus particles. Disruption of host cell cytoskeleton (actin filaments and microtubules) with cytochalasin D and nocodazole showed significant reduction in virus infectivity. Actin filaments are shown to be essential during the initial penetration of the virus across the plasma membrane, whereas microtubules are involved in the trafficking of internalized virus from early endosomes to lysosomes for uncoating. Cells treated with lysosomotropic agents were largely resistant to infection, indicating that a low-pH-dependent step is required for WNV infection. In situ hybridization of DNA probes specific for viral RNA demonstrated the trafficking of uncoated viral RNA genomes to the endoplasmic reticulum.
Figures

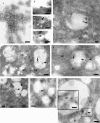

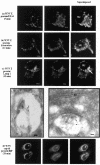
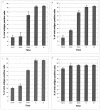
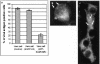


Similar articles
-
Clathrin-mediated endocytosis in living host cells visualized through quantum dot labeling of infectious hematopoietic necrosis virus.J Virol. 2011 Jul;85(13):6252-62. doi: 10.1128/JVI.00109-11. Epub 2011 Apr 27. J Virol. 2011. PMID: 21525360 Free PMC article.
-
Analysis of the endocytic pathway mediating the infectious entry of mosquito-borne flavivirus West Nile into Aedes albopictus mosquito (C6/36) cells.Virology. 2006 Jun 5;349(2):463-75. doi: 10.1016/j.virol.2006.01.022. Epub 2006 Feb 21. Virology. 2006. PMID: 16490225
-
Endocytic pathway followed by dengue virus to infect the mosquito cell line C6/36 HT.Virology. 2008 Aug 15;378(1):193-9. doi: 10.1016/j.virol.2008.05.012. Epub 2008 Jun 20. Virology. 2008. PMID: 18571214
-
The Role of Host Cytoskeleton in Flavivirus Infection.Virol Sin. 2019 Feb;34(1):30-41. doi: 10.1007/s12250-019-00086-4. Epub 2019 Feb 6. Virol Sin. 2019. PMID: 30725318 Free PMC article. Review.
-
Interaction of West Nile and Kunjin viruses with cellular components during morphogenesis.Curr Top Microbiol Immunol. 2002;267:353-72. doi: 10.1007/978-3-642-59403-8_17. Curr Top Microbiol Immunol. 2002. PMID: 12082997 Review. No abstract available.
Cited by
-
Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles.J Virol. 2006 Dec;80(23):11571-8. doi: 10.1128/JVI.01717-06. Epub 2006 Sep 27. J Virol. 2006. PMID: 17005647 Free PMC article.
-
Uptake and transport of novel amphiphilic polyelectrolyte-insulin nanocomplexes by Caco-2 cells--towards oral insulin.Pharm Res. 2011 Apr;28(4):886-96. doi: 10.1007/s11095-010-0345-x. Epub 2011 Jan 7. Pharm Res. 2011. PMID: 21213024
-
Clathrin-mediated endocytosis in living host cells visualized through quantum dot labeling of infectious hematopoietic necrosis virus.J Virol. 2011 Jul;85(13):6252-62. doi: 10.1128/JVI.00109-11. Epub 2011 Apr 27. J Virol. 2011. PMID: 21525360 Free PMC article.
-
Transient cytochalasin-D treatment induces apically administered rAAV2 across tight junctions for transduction of enterocytes.J Gen Virol. 2008 Dec;89(Pt 12):3004-3008. doi: 10.1099/vir.0.2008/001446-0. J Gen Virol. 2008. PMID: 19008386 Free PMC article.
-
Domain I and II from newly emerging goose tembusu virus envelope protein functions as a dominant-negative inhibitor of virus infectivity.Res Vet Sci. 2015 Feb;98:121-6. doi: 10.1016/j.rvsc.2014.11.003. Epub 2014 Nov 18. Res Vet Sci. 2015. PMID: 25481678 Free PMC article.
References
-
- An, S. F., D. Franklin, and K. A. Flemming. 1992. Generation of digoxigenin-labeled double-stranded and single-stranded probes using the polymerase chain reaction. Mol. Cell Probes 6:193-200. - PubMed
-
- Andersen, K. B., and B. A. Nexo. 1983. Entry of murine retrovirus into mouse fibroblasts. Virology 125:85-98. - PubMed
-
- Bayer, N., D. Schober, M. Huttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J. Biol. Chem. 276:3952-3962. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

