Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression
- PMID: 15365188
- PMCID: PMC518848
- DOI: 10.1073/pnas.0405884101
Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression
Abstract
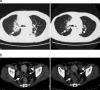
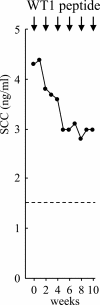
The Wilms' tumor gene WT1 is overexpressed in leukemias and various types of solid tumors, and the WT1 protein was demonstrated to be an attractive target antigen for immunotherapy against these malignancies. Here, we report the outcome of a phase I clinical study of WT1 peptide-based immunotherapy for patients with breast or lung cancer, myelodysplastic syndrome, or acute myeloid leukemia. Patients were intradermally injected with an HLA-A*2402-restricted, natural, or modified 9-mer WT1 peptide emulsified with Montanide ISA51 adjuvant at 0.3, 1.0, or 3.0 mg per body at 2-week intervals, with toxicity and clinical and immunological responses as the principal endpoints. Twenty-six patients received one or more WT1 vaccinations, and 18 of the 26 patients completed WT1 vaccination protocol with three or more injections of WT1 peptides. Toxicity consisted only of local erythema at the WT1 vaccine injection sites in patients with breast or lung cancer or acute myeloid leukemia with adequate normal hematopoiesis, whereas severe leukocytopenia occurred in patients with myelodysplastic syndrome with abnormal hematopoiesis derived from WT1-expressing, transformed hematopoietic stem cells. Twelve of the 20 patients for whom the efficacy of WT1 vaccination could be assessed showed clinical responses such as reduction in leukemic blast cells or tumor sizes and/or tumor markers. A clear correlation was observed between an increase in the frequencies of WT1-specific cytotoxic T lymphocytes after WT1 vaccination and clinical responses. It was therefore demonstrated that WT1 vaccination could induce WT1-specific cytotoxic T lymphocytes and result in cancer regression without damage to normal tissues.
Figures
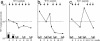
Similar articles
-
Wilms tumor gene peptide-based immunotherapy for patients with overt leukemia from myelodysplastic syndrome (MDS) or MDS with myelofibrosis.Int J Hematol. 2003 Jul;78(1):56-61. doi: 10.1007/BF02983241. Int J Hematol. 2003. PMID: 12894852 Clinical Trial.
-
Identification and characterization of a WT1 (Wilms Tumor Gene) protein-derived HLA-DRB1*0405-restricted 16-mer helper peptide that promotes the induction and activation of WT1-specific cytotoxic T lymphocytes.J Immunother. 2007 Apr;30(3):282-93. doi: 10.1097/01.cji.0000211337.91513.94. J Immunother. 2007. PMID: 17414319
-
WT1 is a tumor-associated antigen in colon cancer that can be recognized by in vitro stimulated cytotoxic T cells.Int J Cancer. 2004 Apr 10;109(3):385-92. doi: 10.1002/ijc.11721. Int J Cancer. 2004. PMID: 14961577
-
WT1 peptide cancer vaccine for patients with hematopoietic malignancies and solid cancers.ScientificWorldJournal. 2007 May 29;7:649-65. doi: 10.1100/tsw.2007.119. ScientificWorldJournal. 2007. PMID: 17619750 Free PMC article. Review.
-
Cancer immunotherapy targeting Wilms' tumor gene WT1 product.Expert Rev Vaccines. 2005 Aug;4(4):503-12. doi: 10.1586/14760584.4.4.503. Expert Rev Vaccines. 2005. PMID: 16117707 Review.
Cited by
-
Improving outcomes in chronic myeloid leukemia through harnessing the immunological landscape.Leukemia. 2021 May;35(5):1229-1242. doi: 10.1038/s41375-021-01238-w. Epub 2021 Apr 8. Leukemia. 2021. PMID: 33833387 Free PMC article. Review.
-
Association of WT1 IgG antibody against WT1 peptide with prolonged survival in glioblastoma multiforme patients vaccinated with WT1 peptide.Int J Cancer. 2016 Sep 15;139(6):1391-401. doi: 10.1002/ijc.30182. Epub 2016 May 31. Int J Cancer. 2016. PMID: 27170523 Free PMC article. Clinical Trial.
-
Lymphodepletion is permissive to the development of spontaneous T-cell responses to the self-antigen PR1 early after allogeneic stem cell transplantation and in patients with acute myeloid leukemia undergoing WT1 peptide vaccination following chemotherapy.Cancer Immunol Immunother. 2012 Jul;61(7):1125-36. doi: 10.1007/s00262-011-1187-z. Epub 2011 Dec 24. Cancer Immunol Immunother. 2012. PMID: 22198310 Free PMC article. Clinical Trial.
-
Regulation of expression of MLAA-34 gene through transcriptional factors E2F1 and MZF-1.Int J Clin Exp Pathol. 2019 Jun 1;12(6):2100-2110. eCollection 2019. Int J Clin Exp Pathol. 2019. PMID: 31934032 Free PMC article.
-
Mapping the HLA ligandome landscape of acute myeloid leukemia: a targeted approach toward peptide-based immunotherapy.Leukemia. 2015 Mar;29(3):647-59. doi: 10.1038/leu.2014.233. Epub 2014 Aug 5. Leukemia. 2015. PMID: 25092142
References
-
- Call, K. M., Glaser, T., Ito, C. Y., Buckler, A. J., Pelletier, J., Haber, D. A., Rose, E. A., Kral, A., Yeger, H., Lewis, W. H., et al. (1990) Cell 60, 509–520. - PubMed
-
- Gessler, M., Poustka, A., Cavenee, W., Neve, R. L., Orkin, S. H. & Bruns, G. A. (1990) Nature 343, 774–778. - PubMed
-
- Drummond, I. A., Madden, S. L., Rohwer-Nutter, P., Bell, G. I., Sukhatme, V. P. & Rauscher, F. J., III (1992) Science 257, 674–678. - PubMed
-
- Godyer, P., Dehbi, M., Torban, E., Bruening, W. & Pelletier, J. (1995) Oncogene 10, 1125–1129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

