Glucose-stimulated insulin response in pregnant sheep following acute suppression of plasma non-esterified fatty acid concentrations
- PMID: 15352999
- PMCID: PMC519029
- DOI: 10.1186/1477-7827-2-64
Glucose-stimulated insulin response in pregnant sheep following acute suppression of plasma non-esterified fatty acid concentrations
Abstract
Background: Elevated non-esterified fatty acids (NEFA) concentrations in non-pregnant animals have been reported to decrease pancreatic responsiveness. As ovine gestation advances, maternal insulin concentrations fall and NEFA concentrations increase. Experiments were designed to examine if the pregnancy-associated rise in NEFA concentration is associated with a reduced pancreatic sensitivity to glucose in vivo. We investigated the possible relationship of NEFA concentrations in regulating maternal insulin concentrations during ovine pregnancy at three physiological states, non-pregnant, non-lactating (NPNL), 105 and 135 days gestational age (dGA, term 147+/- 3 days).
Methods: The plasma concentrations of insulin, growth hormone (GH) and ovine placental lactogen (oPL) were determined by double antibody radioimmunoassay. Insulin responsiveness to glucose was measured using bolus injection and hyperglycaemic clamp techniques in 15 non-pregnant, non-lactating ewes and in nine pregnant ewes at 105 dGA and near term at 135 dGA. Plasma samples were also collected for hormone determination. In addition to bolus injection glucose and insulin Area Under Curve calculations, the Mean Plasma Glucose Increment, Glucose Infusion Rate and Mean Plasma Insulin Increment and Area Under Curve were determined for the hyperglycaemic clamp procedures. Statistical analysis of data was conducted with Students t-tests, repeated measures ANOVA and 2-way ANOVA.
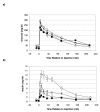
Results: Maternal growth hormone, placental lactogen and NEFA concentrations increased, while basal glucose and insulin concentrations declined with advancing gestation. At 135 dGA following bolus glucose injections, peak insulin concentrations and insulin area under curve (AUC) profiles were significantly reduced in pregnant ewes compared with NPNL control ewes (p < 0.001 and P < 0.001, respectively). In hyperglycaemic clamp studies, while maintaining glucose levels not different from NPNL ewes, pregnant ewes displayed significantly reduced insulin responses and a maintained depressed insulin secretion. In NPNL ewes, 105 and 135 dGA ewes, the Glucose Infusion Rate (GIR) was constant at approximately 5.8 mg glucose/kg/min during the last 40 minutes of the hyperglycaemic clamp and the Mean Plasma Insulin Increment (MPII) was only significantly (p < 0.001) greater in NPNL ewes. Following the clamp, NEFA concentrations were reduced by approximately 60% of pre-clamp levels in all groups, though a blunted and suppressed insulin response was maintained in 105 and 135 dGA ewes.
Conclusions: Results suggest that despite an acute suppression of circulating NEFA concentrations during pregnancy, the associated steroids and hormones of pregnancy and possibly NEFA metabolism, may act to maintain a reduced insulin output, thereby sparing glucose for non-insulin dependent placental uptake and ultimately, fetal requirements.
Figures

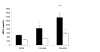
Similar articles
-
Comparative effects of recombinant ovine placental lactogen and bovine growth hormone on galactopoiesis in ewes.J Dairy Sci. 1997 Apr;80(4):640-5. doi: 10.3168/jds.S0022-0302(97)75982-4. J Dairy Sci. 1997. PMID: 9149958
-
[Effect of insulin on glucose and and fat metabolism in ewes during various reproductive states in normal and hypocalcemia].Dtsch Tierarztl Wochenschr. 1997 Sep;104(9):359-65. Dtsch Tierarztl Wochenschr. 1997. PMID: 9410723 German.
-
Post-glucose load changes of plasma key metabolite and insulin concentrations during pregnancy and lactation in ewes with different susceptibility to pregnancy toxaemia.J Anim Physiol Anim Nutr (Berl). 2013 Oct;97(5):971-85. doi: 10.1111/jpn.12010. Epub 2012 Oct 6. J Anim Physiol Anim Nutr (Berl). 2013. PMID: 23039765
-
Pregnancy but not moderate undernutrition attenuates insulin suppression of fat mobilization in sheep.J Nutr. 1994 Dec;124(12):2431-6. doi: 10.1093/jn/124.12.431. J Nutr. 1994. PMID: 16856324
-
Effects of continuous intravenous infusion of an ovine placental extract enriched in placental lactogen on plasma hormones, metabolites and metabolite biokinetics in non-pregnant sheep.J Endocrinol. 1987 May;113(2):277-83. doi: 10.1677/joe.0.1130277. J Endocrinol. 1987. PMID: 3295103
Cited by
-
Colostrum traits and newborn body weight and growth: comparison between single and twin underfed sheep pregnancies.Front Vet Sci. 2023 Sep 8;10:1256989. doi: 10.3389/fvets.2023.1256989. eCollection 2023. Front Vet Sci. 2023. PMID: 37745219 Free PMC article.
-
Fluctuations of serum cortisol, insulin and non-esterified fatty acid concentrations in growing ewes over the year.Ir Vet J. 2014 Oct 18;67(1):22. doi: 10.1186/2046-0481-67-22. eCollection 2014. Ir Vet J. 2014. PMID: 25337389 Free PMC article.
-
Lactogenic activity of rats stimulated by Gunnera perpensa L. (Gunneraceae) from South Africa.Afr J Tradit Complement Altern Med. 2012 Jul 1;9(4):561-73. doi: 10.4314/ajtcam.v9i4.14. eCollection 2012. Afr J Tradit Complement Altern Med. 2012. PMID: 23983393 Free PMC article.
-
A Randomized Crossover Intervention Study on the Effect a Standardized Maté Extract (Ilex paraguariensis A. St.-Hil.) in Men Predisposed to Cardiovascular Risk.Nutrients. 2020 Dec 23;13(1):14. doi: 10.3390/nu13010014. Nutrients. 2020. PMID: 33374524 Free PMC article. Clinical Trial.
-
Elevated maternal cortisol leads to relative maternal hyperglycemia and increased stillbirth in ovine pregnancy.Am J Physiol Regul Integr Comp Physiol. 2014 Aug 15;307(4):R405-13. doi: 10.1152/ajpregu.00530.2013. Epub 2014 Jun 11. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24920731 Free PMC article.
References
-
- Bell AW. Pregnancy and fetal metabolism. In: Forbes JM, France JM, editor. In Quantitative aspects of ruminant digestion and metabolism. Oxford UK: CAB International; 1993. pp. 406–431.
-
- Blom AK, Hove K, Nedkvitne JJ. Plasma insulin and growth hormone concentrations in pregnant sheep II: Post-absorptive levels in mid- and late pregnancy. Acta Endocrinologica. 1976;82:553–560. - PubMed
-
- Hove K, Blom AK. Plasma insulin and growth hormone concentrations in pregnant sheep I: Diurnal variations in mid- and late pregnancy. Acta Endocrinologica. 1976;82:544–552. - PubMed
-
- Fowden AL. The effect of twin pregnancy on insulin secretion in the ewe. Journal of Endocrinology. 1982;94:65P.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

