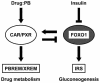
Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes
- PMID: 15340055
- PMCID: PMC515037
- DOI: 10.1128/MCB.24.18.7931-7940.2004
Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes
Abstract
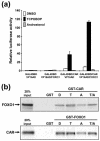
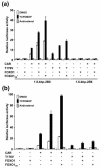
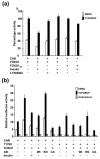
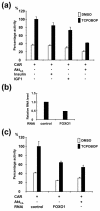
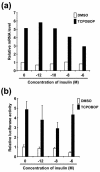
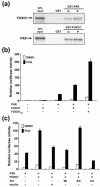
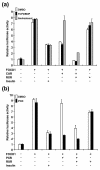
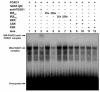
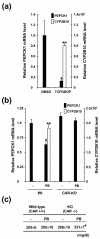
The nuclear receptors CAR and PXR activate hepatic genes in response to therapeutic drugs and xenobiotics, leading to the induction of drug-metabolizing enzymes, such as cytochrome P450. Insulin inhibits the ability of FOXO1 to express genes encoding gluconeogenic enzymes. Induction by drugs is known to be decreased by insulin, whereas gluconeogenic activity is often repressed by treatment with certain drugs, such as phenobarbital (PB). Performing cell-based transfection assays with drug-responsive and insulin-responsive enhancers, glutathione S-transferase pull down, RNA interference (RNAi), and mouse primary hepatocytes, we examined the molecular mechanism by which nuclear receptors and FOXO1 could coordinately regulate both enzyme pathways. FOXO1 was found to be a coactivator to CAR- and PXR-mediated transcription. In contrast, CAR and PXR, acting as corepressors, downregulated FOXO1-mediated transcription in the presence of their activators, such as 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) and pregnenolone 16alpha-carbonitrile, respectively. A constitutively active mutant of the insulin-responsive protein kinase Akt, but not the kinase-negative mutant, effectively blocked FOXO1 activity in cell-based assays. Thus, insulin could repress the receptors by activating the Akt-FOXO1 signal, whereas drugs could interfere with FOXO1-mediated transcription by activating CAR and/or PXR. Treatment with TCPOBOP or PB decreased the levels of phosphoenolpyruvate carboxykinase 1 mRNA in mice but not in Car(-/-) mice. We conclude that FOXO1 and the nuclear receptors reciprocally coregulate their target genes, modulating both drug metabolism and gluconeogenesis.
Figures
Similar articles
-
Regulatory cross-talk between drug metabolism and lipid homeostasis: constitutive androstane receptor and pregnane X receptor increase Insig-1 expression.Mol Pharmacol. 2008 Apr;73(4):1282-9. doi: 10.1124/mol.107.041012. Epub 2008 Jan 10. Mol Pharmacol. 2008. PMID: 18187584
-
Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways.Drug Metab Dispos. 2005 Sep;33(9):1276-82. doi: 10.1124/dmd.105.003988. Epub 2005 May 26. Drug Metab Dispos. 2005. PMID: 15919853
-
RNA-Seq reveals common and unique PXR- and CAR-target gene signatures in the mouse liver transcriptome.Biochim Biophys Acta. 2016 Sep;1859(9):1198-1217. doi: 10.1016/j.bbagrm.2016.04.010. Epub 2016 Apr 23. Biochim Biophys Acta. 2016. PMID: 27113289 Free PMC article.
-
The roles of nuclear receptors CAR and PXR in hepatic energy metabolism.Drug Metab Pharmacokinet. 2008;23(1):8-13. doi: 10.2133/dmpk.23.8. Drug Metab Pharmacokinet. 2008. PMID: 18305370 Review.
-
P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR.Arch Biochem Biophys. 1999 Sep 1;369(1):11-23. doi: 10.1006/abbi.1999.1351. Arch Biochem Biophys. 1999. PMID: 10462436 Review.
Cited by
-
The constitutive androstane receptor activator 4-[(4R,6R)-4,6-diphenyl-1,3-dioxan-2-yl]-N,N-dimethylaniline inhibits the gluconeogenic genes PEPCK and G6Pase through the suppression of HNF4α and FOXO1 transcriptional activity.Br J Pharmacol. 2013 Apr;168(8):1923-32. doi: 10.1111/bph.12090. Br J Pharmacol. 2013. PMID: 23231652 Free PMC article.
-
Targeting xenobiotic receptors PXR and CAR for metabolic diseases.Trends Pharmacol Sci. 2012 Oct;33(10):552-8. doi: 10.1016/j.tips.2012.07.003. Epub 2012 Aug 10. Trends Pharmacol Sci. 2012. PMID: 22889594 Free PMC article. Review.
-
The orphan nuclear receptor DAX-1 functions as a potent corepressor of the constitutive androstane receptor (NR1I3).Mol Pharmacol. 2012 Nov;82(5):918-28. doi: 10.1124/mol.112.080721. Epub 2012 Aug 15. Mol Pharmacol. 2012. PMID: 22896671 Free PMC article.
-
Insights into CYP2B6-mediated drug-drug interactions.Acta Pharm Sin B. 2016 Sep;6(5):413-425. doi: 10.1016/j.apsb.2016.07.016. Epub 2016 Aug 9. Acta Pharm Sin B. 2016. PMID: 27709010 Free PMC article. Review.
-
Nuclear receptor CAR represses TNFalpha-induced cell death by interacting with the anti-apoptotic GADD45B.PLoS One. 2010 Apr 12;5(4):e10121. doi: 10.1371/journal.pone.0010121. PLoS One. 2010. PMID: 20404936 Free PMC article.
References
-
- Dixon, R. L., L. G. Hart, and J. R. Fouts. 1961. The metabolism of drugs by liver microsomes from alloxan-diabetic rats. J. Pharmacol. Exp. Ther. 133:7-11. - PubMed
-
- Dowell, P., T. C. Otto, S. Adi, and M. D. Lane. 2003. Convergence of proxisome proliferator-activated receptor-γ and Foxo1 signaling pathway. J. Biol. Chem. 278:45485-45491. - PubMed
-
- Goodwin, B., M. R. Redinbo, and S. A. Kliewer. 2002. Regulation of cyp3a gene transcription by the pregnane x receptor. Annu. Rev. Pharmacol. Toxicol. 42:1-23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
