Role of protein kinase C delta in reactivation of Kaposi's sarcoma-associated herpesvirus
- PMID: 15331751
- PMCID: PMC515025
- DOI: 10.1128/JVI.78.18.10187-10192.2004
Role of protein kinase C delta in reactivation of Kaposi's sarcoma-associated herpesvirus
Abstract
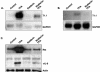
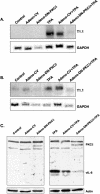
TPA (12-O-tetradecanoylphorbol-13-acetate), a well-known activator of protein kinase C (PKC), can experimentally induce reactivation of Kaposi's sarcoma-associated herpesvirus (KSHV) in certain latently infected cells. We selectively blocked the activity of PKC isoforms by using GF 109203X or rottlerin and demonstrated that this inhibition largely decreased lytic KSHV reactivation by TPA. Translocation of the PKCdelta isoform was evident shortly after TPA stimulation. Overexpression of the dominant-negative PKCdelta mutant supported an essential role for the PKCdelta isoform in virus reactivation, yet overexpression of PKCdelta alone was not sufficient to induce lytic reactivation of KSHV, suggesting that additional signaling molecules participate in this pathway.
Copyright 2004 American Society for Microbiology
Figures



Similar articles
-
An essential role of ERK signalling in TPA-induced reactivation of Kaposi's sarcoma-associated herpesvirus.J Gen Virol. 2006 Apr;87(Pt 4):795-802. doi: 10.1099/vir.0.81619-0. J Gen Virol. 2006. PMID: 16528027
-
De novo protein synthesis is required for lytic cycle reactivation of Epstein-Barr virus, but not Kaposi's sarcoma-associated herpesvirus, in response to histone deacetylase inhibitors and protein kinase C agonists.J Virol. 2007 Sep;81(17):9279-91. doi: 10.1128/JVI.00982-07. Epub 2007 Jun 27. J Virol. 2007. PMID: 17596302 Free PMC article.
-
Oxidant species are involved in T/B-mediated ERK1/2 phosphorylation that activates p53-p21 axis to promote KSHV lytic cycle in PEL cells.Free Radic Biol Med. 2017 Nov;112:327-335. doi: 10.1016/j.freeradbiomed.2017.08.005. Epub 2017 Aug 8. Free Radic Biol Med. 2017. PMID: 28801242
-
Targeted inhibition of calcineurin signaling blocks calcium-dependent reactivation of Kaposi sarcoma-associated herpesvirus.Blood. 2001 Apr 15;97(8):2374-80. doi: 10.1182/blood.v97.8.2374. Blood. 2001. PMID: 11290600
-
Effect of protein kinase C activation on intracellular Ca2+ signaling and integrity of intestinal epithelial cells.Eur J Pharmacol. 2005 Jul 25;518(1):1-9. doi: 10.1016/j.ejphar.2005.06.008. Eur J Pharmacol. 2005. PMID: 16005455
Cited by
-
Molecular biology of KSHV lytic reactivation.Viruses. 2015 Jan 14;7(1):116-53. doi: 10.3390/v7010116. Viruses. 2015. PMID: 25594835 Free PMC article. Review.
-
Protein kinase C-epsilon regulates the apoptosis and survival of glioma cells.Cancer Res. 2005 Aug 15;65(16):7301-9. doi: 10.1158/0008-5472.CAN-05-1064. Cancer Res. 2005. PMID: 16103081 Free PMC article.
-
Molecular biology of KSHV in relation to AIDS-associated oncogenesis.Cancer Treat Res. 2007;133:69-127. doi: 10.1007/978-0-387-46816-7_3. Cancer Treat Res. 2007. PMID: 17672038 Free PMC article. Review.
-
The inflammatory kinase MAP4K4 promotes reactivation of Kaposi's sarcoma herpesvirus and enhances the invasiveness of infected endothelial cells.PLoS Pathog. 2013;9(11):e1003737. doi: 10.1371/journal.ppat.1003737. Epub 2013 Nov 7. PLoS Pathog. 2013. PMID: 24244164 Free PMC article. Clinical Trial.
-
Regulation of Autophagy in Cells Infected With Oncogenic Human Viruses and Its Impact on Cancer Development.Front Cell Dev Biol. 2020 Feb 28;8:47. doi: 10.3389/fcell.2020.00047. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32181249 Free PMC article. Review.
References
-
- Antman, K., and Y. Chang. 2000. Kaposi's sarcoma. N. Engl. J. Med. 342:1027-1038. - PubMed
-
- Ashendel, C. L., J. M. Staller, and R. K. Boutwell. 1983. Protein kinase activity associated with a phorbol ester receptor purified from mouse brain. Cancer Res. 43:4333-4337. - PubMed
-
- Baumann, M., O. Gires, W. Kolch, H. Mischak, R. Zeidler, D. Pich, and W. Hammerschmidt. 2000. The PKC targeting protein RACK1 interacts with the Epstein-Barr virus activator protein BZLF1. Eur. J. Biochem. 267:3891-3901. - PubMed
-
- Boshoff, C., S. J. Gao, L. E. Healy, S. Matthews, A. J. Thomas, L. Coignet, R. A. Warnke, J. A. Strauchen, E. Matutes, O. W. Kamel, P. S. Moore, R. A. Weiss, and Y. Chang. 1998. Establishing a KSHV+ cell line (BCP-1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood 91:1671-1679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

