LEDGF/p75 determines cellular trafficking of diverse lentiviral but not murine oncoretroviral integrase proteins and is a component of functional lentiviral preintegration complexes
- PMID: 15308744
- PMCID: PMC506940
- DOI: 10.1128/JVI.78.17.9524-9537.2004
LEDGF/p75 determines cellular trafficking of diverse lentiviral but not murine oncoretroviral integrase proteins and is a component of functional lentiviral preintegration complexes
Abstract
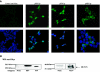
Human immunodeficiency virus type 1 (HIV-1), feline immunodeficiency virus (FIV), and Moloney murine leukemia virus (MoMLV) integrases were stably expressed to determine their intracellular trafficking. Each lentiviral integrase localized to cell nuclei in close association with chromatin while the murine oncoretroviral integrase was cytoplasmic. Fusions of pyruvate kinase to the lentiviral integrases did not reveal transferable nuclear localization signals. The intracellular trafficking of each was determined instead by the transcriptional coactivator LEDGF/p75, which was required for nuclear localization. Stable small interfering RNA expression eliminated detectable LEDGF/p75 expression and caused dramatic, stable redistribution of each lentiviral integrase from nucleus to cytoplasm while the distribution of MoMLV integrase was unaffected. In addition, endogenous LEDGF/p75 coimmunoprecipitated specifically with each lentiviral integrase. In vitro integration assays with preintegration complexes (PICs) showed that endogenous LEDGF/p75 is a component of functional HIV-1 and FIV PICs. However, HIV-1 and FIV infection and replication in LEDGF/p75-deficient cells was equivalent to that in control cells, whether cells were dividing or growth arrested. Two-long terminal repeat circle accumulation in nondividing cell nuclei was also equivalent to that of LEDGF/p75 wild-type cells. Virions produced in LEDGF/p75-deficient cells had normal infectivity. We conclude that LEDGF/p75 fully accounts for cellular trafficking of diverse lentiviral, but not oncoretroviral, integrases and is the main lentiviral integrase-to-chromatin tethering factor. While lentiviral PIC nuclear import is unaffected by LEDGF/p75 knockdown, this protein is a component of functional lentiviral PICs. A role in HIV-1 integration site distribution merits investigation.
Figures







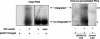


Similar articles
-
Identification of the LEDGF/p75 HIV-1 integrase-interaction domain and NLS reveals NLS-independent chromatin tethering.J Cell Sci. 2005 Apr 15;118(Pt 8):1733-43. doi: 10.1242/jcs.02299. Epub 2005 Mar 29. J Cell Sci. 2005. PMID: 15797927
-
Virus evolution reveals an exclusive role for LEDGF/p75 in chromosomal tethering of HIV.PLoS Pathog. 2007 Mar;3(3):e47. doi: 10.1371/journal.ppat.0030047. PLoS Pathog. 2007. PMID: 17397262 Free PMC article.
-
Lens epithelium-derived growth factor/p75 prevents proteasomal degradation of HIV-1 integrase.J Biol Chem. 2004 Dec 31;279(53):55570-7. doi: 10.1074/jbc.M408508200. Epub 2004 Oct 8. J Biol Chem. 2004. PMID: 15475359
-
The lentiviral integrase binding protein LEDGF/p75 and HIV-1 replication.PLoS Pathog. 2008 Mar 28;4(3):e1000046. doi: 10.1371/journal.ppat.1000046. PLoS Pathog. 2008. PMID: 18369482 Free PMC article. Review.
-
The LEDGF/p75 integrase interaction, a novel target for anti-HIV therapy.Virology. 2013 Jan 5;435(1):102-9. doi: 10.1016/j.virol.2012.09.033. Virology. 2013. PMID: 23217620 Review.
Cited by
-
HIV-1 Maturation: Lessons Learned from Inhibitors.Viruses. 2020 Aug 26;12(9):940. doi: 10.3390/v12090940. Viruses. 2020. PMID: 32858867 Free PMC article. Review.
-
LEDGF/p75-independent HIV-1 replication demonstrates a role for HRP-2 and remains sensitive to inhibition by LEDGINs.PLoS Pathog. 2012;8(3):e1002558. doi: 10.1371/journal.ppat.1002558. Epub 2012 Mar 1. PLoS Pathog. 2012. PMID: 22396646 Free PMC article.
-
A protein ballet around the viral genome orchestrated by HIV-1 reverse transcriptase leads to an architectural switch: from nucleocapsid-condensed RNA to Vpr-bridged DNA.Virus Res. 2013 Feb;171(2):287-303. doi: 10.1016/j.virusres.2012.09.008. Epub 2012 Sep 24. Virus Res. 2013. PMID: 23017337 Free PMC article.
-
Retroviral DNA Integration.Chem Rev. 2016 Oct 26;116(20):12730-12757. doi: 10.1021/acs.chemrev.6b00125. Epub 2016 May 20. Chem Rev. 2016. PMID: 27198982 Free PMC article. Review.
-
Mouse mammary tumor virus integration site selection in human and mouse genomes.J Virol. 2008 Feb;82(3):1360-7. doi: 10.1128/JVI.02098-07. Epub 2007 Nov 21. J Virol. 2008. PMID: 18032509 Free PMC article.
References
-
- Bouyac-Bertoia, M., J. Dvorin, R. Fouchier, Y. Jenkins, B. Meyer, L. Wu, M. Emerman, and M. H. Malim. 2001. HIV-1 infection requires a functional integrase NLS. Mol. Cell 7:1025-1035. - PubMed
-
- Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. - PubMed
-
- Bushman, F. D. 1999. Host proteins in retroviral cDNA integration. Adv. Virus Res. 52:301-317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

