Human cytomegalovirus UL84 oligomerization and heterodimerization domains act as transdominant inhibitors of oriLyt-dependent DNA replication: evidence that IE2-UL84 and UL84-UL84 interactions are required for lytic DNA replication
- PMID: 15308715
- PMCID: PMC506931
- DOI: 10.1128/JVI.78.17.9203-9214.2004
Human cytomegalovirus UL84 oligomerization and heterodimerization domains act as transdominant inhibitors of oriLyt-dependent DNA replication: evidence that IE2-UL84 and UL84-UL84 interactions are required for lytic DNA replication
Abstract
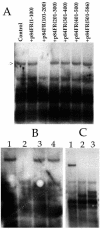
Human cytomegalovirus (HCMV) UL84 encodes a 75-kDa protein required for oriLyt-dependent DNA replication and interacts with IE2 in infected and transfected cells. UL84 localizes to the nucleus of transfected and infected cells and is found in viral replication compartments. In transient assays it was shown that UL84 can interfere with the IE2-mediated transactivation of the UL112/113 promoter of HCMV. To determine whether UL84 protein-protein interactions are necessary for lytic DNA synthesis, we purified UL84 and used this protein to generate a monoclonal antibody. Using this antibody, we now show that UL84 forms a stable interaction with itself in vivo. The point of self-interaction maps to a region of the protein between amino acids 151 and 200, a domain that contains a series of highly charged amino acid residues. Coimmunoprecipitation assays determined that UL84 interacts with a protein domain present within the first 215 amino acids of IE2. We also show that an intact leucine zipper domain of UL84 is required for a stable interaction with IE2 and UL84 leucine zipper mutants fail to complement oriLyt-dependent DNA replication. UL84 leucine zipper mutants no longer interfere with IE2-mediated transactivation of the UL112/113 promoter, confirming that the leucine zipper is essential for a functional interaction with IE2. In addition, we demonstrate that both the leucine zipper and oligomerization domains of UL84 can act as transdominant-negative inhibitors of lytic replication in the transient assay, strongly suggesting that both an IE2-UL84 and a UL84-UL84 interaction are required for DNA synthesis.
Figures



Similar articles
-
Characteristics of Immediate-Early 2 (IE2) and UL84 Proteins in UL84-Independent Strains of Human Cytomegalovirus (HCMV).Microbiol Spectr. 2021 Oct 31;9(2):e0053921. doi: 10.1128/Spectrum.00539-21. Epub 2021 Sep 22. Microbiol Spectr. 2021. PMID: 34550009 Free PMC article.
-
Human cytomegalovirus DNA replication requires transcriptional activation via an IE2- and UL84-responsive bidirectional promoter element within oriLyt.J Virol. 2004 Nov;78(21):11664-77. doi: 10.1128/JVI.78.21.11664-11677.2004. J Virol. 2004. PMID: 15479808 Free PMC article.
-
Human cytomegalovirus UL84 interacts with an RNA stem-loop sequence found within the RNA/DNA hybrid region of oriLyt.J Virol. 2007 Jul;81(13):7077-85. doi: 10.1128/JVI.00058-07. Epub 2007 Apr 25. J Virol. 2007. PMID: 17459920 Free PMC article.
-
Evidence that the UL84 gene product of human cytomegalovirus is essential for promoting oriLyt-dependent DNA replication and formation of replication compartments in cotransfection assays.J Virol. 1996 Nov;70(11):7398-413. doi: 10.1128/JVI.70.11.7398-7413.1996. J Virol. 1996. PMID: 8892858 Free PMC article.
-
Nuts and bolts of human cytomegalovirus lytic DNA replication.Curr Top Microbiol Immunol. 2008;325:153-66. doi: 10.1007/978-3-540-77349-8_9. Curr Top Microbiol Immunol. 2008. PMID: 18637505 Review.
Cited by
-
Insights into the Transcriptome of Human Cytomegalovirus: A Comprehensive Review.Viruses. 2023 Aug 8;15(8):1703. doi: 10.3390/v15081703. Viruses. 2023. PMID: 37632045 Free PMC article. Review.
-
Identification of cellular proteins associated with human cytomegalovirus (HCMV) DNA replication suggests novel cellular and viral interactions.Virology. 2022 Jan;566:26-41. doi: 10.1016/j.virol.2021.11.004. Epub 2021 Nov 22. Virology. 2022. PMID: 34861458 Free PMC article.
-
Characteristics of Immediate-Early 2 (IE2) and UL84 Proteins in UL84-Independent Strains of Human Cytomegalovirus (HCMV).Microbiol Spectr. 2021 Oct 31;9(2):e0053921. doi: 10.1128/Spectrum.00539-21. Epub 2021 Sep 22. Microbiol Spectr. 2021. PMID: 34550009 Free PMC article.
-
Casein kinase-2-mediated phosphorylation increases the SUMO-dependent activity of the cytomegalovirus transactivator IE2.J Biol Chem. 2019 Oct 4;294(40):14546-14561. doi: 10.1074/jbc.RA119.009601. Epub 2019 Aug 1. J Biol Chem. 2019. PMID: 31371453 Free PMC article.
-
Requirement of the N-terminal residues of human cytomegalovirus UL112-113 proteins for viral growth and oriLyt-dependent DNA replication.J Microbiol. 2015 Aug;53(8):561-9. doi: 10.1007/s12275-015-5301-3. Epub 2015 Jul 31. J Microbiol. 2015. PMID: 26224459
References
-
- AuCoin, D. P., K. S. Colletti, S. A. Cei, I. Papouskova, M. Tarrant, and G. S. Pari. 2004. Amplification of the Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 lytic origin of DNA replication is dependent upon a cis-acting AT-rich region and an ORF50 response element and the trans-acting factors ORF50 (K-Rta) and K8 (K-bZIP). Virology 318:542-555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

