TIP27: a novel repressor of the nuclear orphan receptor TAK1/TR4
- PMID: 15302918
- PMCID: PMC514368
- DOI: 10.1093/nar/gkh741
TIP27: a novel repressor of the nuclear orphan receptor TAK1/TR4
Abstract
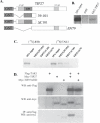
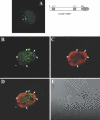
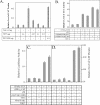
The nuclear orphan receptor TAK1/TR4 functions as a positive as well as a negative regulator of transcription; however, little is known about the factors regulating or mediating its activity. Yeast two-hybrid analysis using the ligand-binding domain (LBD) of TAK1 as bait identified a novel TAK1-interacting protein, referred to as TIP27, which functions as a repressor of TAK1-mediated transactivation. TIP27 is a 27 kDa protein containing two zinc finger motifs. Mammalian two-hybrid analysis showed that TIP27 interacts specifically with TAK1 and not with several other nuclear receptors tested. The region between Asp39 and Lys79 of TIP27, referred to as TAK1-interaction domain (TID), is critical for its interaction with TAK1 while the TAK1-LBD from helix 3 until the C-terminus is required for the optimal interaction with TIP27. Pull-down assays demonstrated that the TIP27 physically interacts with TAK1 and supported the critical importance of the TID. Confocal microscopy showed that in the nucleus, TIP27 and TAK1 co-localize. TIP27 acts as a strong repressor of DR1-dependent transcriptional activation by TAK1. This repression does not involve the inhibition of TAK1 homodimerization or DR1 binding but may be due to an effect on co-activator recruitment by TAK1. Our results indicate that TIP27 functions as a TAK1-selective repressor.
Figures





Similar articles
-
Identification of a novel testicular orphan receptor-4 (TR4)-associated protein as repressor for the selective suppression of TR4-mediated transactivation.J Biol Chem. 2003 Feb 28;278(9):7709-17. doi: 10.1074/jbc.M207116200. Epub 2002 Dec 16. J Biol Chem. 2003. PMID: 12486131
-
The orphan receptor TAK1 acts as a repressor of RAR-, RXR- and T3R-mediated signaling pathways.Biochem Biophys Res Commun. 1995 Jun 6;211(1):83-91. doi: 10.1006/bbrc.1995.1781. Biochem Biophys Res Commun. 1995. PMID: 7779113
-
Regulation of peroxisome proliferator-activated receptor alpha-induced transactivation by the nuclear orphan receptor TAK1/TR4.J Biol Chem. 1998 May 1;273(18):10948-57. doi: 10.1074/jbc.273.18.10948. J Biol Chem. 1998. PMID: 9556573
-
Gene silencing by nuclear orphan receptors.Vitam Horm. 2004;68:1-48. doi: 10.1016/S0083-6729(04)68001-0. Vitam Horm. 2004. PMID: 15193450 Review.
-
Modulation of thyroid hormone receptor silencing function by co-repressors and a synergizing transcription factor.Biochem Soc Trans. 2000;28(4):386-9. Biochem Soc Trans. 2000. PMID: 10961925 Review.
Cited by
-
CircRNA ITCH Inhibits Epithelial-Mesenchymal Transformation and Promotes Apoptosis in Papillary Thyroid Carcinoma via miR-106a-5p/JAZF1 Axis.Biochem Genet. 2024 Dec;62(6):4755-4769. doi: 10.1007/s10528-024-10672-1. Epub 2024 Feb 15. Biochem Genet. 2024. PMID: 38358587 Free PMC article.
-
JAZF1 Suppresses Papillary Thyroid Carcinoma Cell Proliferation and Facilitates Apoptosis via Regulating TAK1/NF-κB Pathways.Onco Targets Ther. 2019 Dec 2;12:10501-10514. doi: 10.2147/OTT.S230597. eCollection 2019. Onco Targets Ther. 2019. PMID: 31819531 Free PMC article.
-
Minireview: Pathophysiological roles of the TR4 nuclear receptor: lessons learned from mice lacking TR4.Mol Endocrinol. 2014 Jun;28(6):805-21. doi: 10.1210/me.2013-1422. Epub 2014 Apr 4. Mol Endocrinol. 2014. PMID: 24702179 Free PMC article.
-
A genetic link between type 2 diabetes and prostate cancer.Diabetologia. 2008 Oct;51(10):1757-60. doi: 10.1007/s00125-008-1114-9. Epub 2008 Aug 12. Diabetologia. 2008. PMID: 18696045
-
JAZF1/SUZ12 gene fusion in endometrial stromal sarcomas.Orphanet J Rare Dis. 2016 Feb 16;11:15. doi: 10.1186/s13023-016-0400-8. Orphanet J Rare Dis. 2016. PMID: 26879382 Free PMC article. Review.
References
-
- Hirose T., Apfel,R., Pfahl,M. and Jetten,A.M. (1995) The orphan receptor TAK1 acts as a repressor of RAR-, RXR- and T3R-mediated signaling pathways. Biochem. Biophys. Res. Commun., 211, 83–91. - PubMed
-
- Chang C., Kokontis,J., Acakpo-Satchivi,L., Liao,S., Takeda,H. and Chang,Y. (1989) Molecular cloning of new human TR2 receptors: a class of steroid receptor with multiple ligand-binding domains. Biochem. Biophys. Res. Commun., 165, 735–741. - PubMed
-
- Hirose T., O'Brien,D.A. and Jetten,A.M. (1995) Cloning of the gene encoding the murine orphan receptor TAK1 and cell-type-specific expression in testis. Gene, 163, 239–242. - PubMed
-
- Laudet V. (1997) Evolution of the nuclear receptor superfamily: early diversification from an ancestral orphan receptor. J. Mol. Endocrinol., 19, 207–226. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

