Role of an inducible single-domain hemoglobin in mediating resistance to nitric oxide and nitrosative stress in Campylobacter jejuni and Campylobacter coli
- PMID: 15292134
- PMCID: PMC490904
- DOI: 10.1128/JB.186.16.5332-5341.2004
Role of an inducible single-domain hemoglobin in mediating resistance to nitric oxide and nitrosative stress in Campylobacter jejuni and Campylobacter coli
Abstract
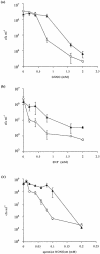
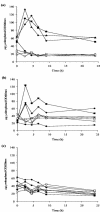
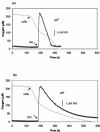
Campylobacter jejuni expresses two hemoglobins, each of which exhibits a heme pocket and structural signatures in common with vertebrate and plant globins. One of these, designated Cgb, is homologous to Vgb from Vitreoscilla stercoraria and does not possess the reductase domain seen in the flavohemoglobins. A Cgb-deficient mutant of C. jejuni was hypersensitive to nitrosating agents (S-nitrosoglutathione [GSNO] or sodium nitroprusside) and a nitric oxide-releasing compound (spermine NONOate). The sensitivity of the Cgb-deficient mutant to methyl viologen, hydrogen peroxide, and organic peroxides, however, was the same as for the wild type. Consistent with the protective role of Cgb against NO-related stress, cgb expression was minimal in standard laboratory media but strongly and specifically induced after exposure to nitrosative stress. In contrast, the expression of Cgb was independent of aeration and the presence of superoxide. In the absence of preinduction by exposure to nitrosative stress, no difference was seen in the degree of respiratory inhibition by NO or the half-life of the NO signal when cells of the wild type and the cgb mutant were compared. However, cells expressing GSNO-upregulated levels of Cgb exhibited robust NO consumption and respiration that was relatively NO insensitive compared to the respiration of the cgb mutant. Based on similar studies in Campylobacter coli, we also propose an identical role for Cgb in this closely related species. We conclude that, unlike the archetypal single-domain globin Vgb, Cgb forms a specific and inducible defense against NO and nitrosating agents.
Figures

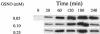
Similar articles
-
Nitric oxide reactivities of the two globins of the foodborne pathogen Campylobacter jejuni: roles in protection from nitrosative stress and analysis of potential reductants.Nitric Oxide. 2013 Nov 1;34:65-75. doi: 10.1016/j.niox.2013.06.002. Epub 2013 Jun 11. Nitric Oxide. 2013. PMID: 23764490
-
NssR, a member of the Crp-Fnr superfamily from Campylobacter jejuni, regulates a nitrosative stress-responsive regulon that includes both a single-domain and a truncated haemoglobin.Mol Microbiol. 2005 Aug;57(3):735-50. doi: 10.1111/j.1365-2958.2005.04723.x. Mol Microbiol. 2005. PMID: 16045618
-
Growth of Campylobacter jejuni on nitrate and nitrite: electron transport to NapA and NrfA via NrfH and distinct roles for NrfA and the globin Cgb in protection against nitrosative stress.Mol Microbiol. 2007 Jan;63(2):575-90. doi: 10.1111/j.1365-2958.2006.05532.x. Mol Microbiol. 2007. PMID: 17241202
-
The globins of Campylobacter jejuni.Adv Microb Physiol. 2013;63:97-145. doi: 10.1016/B978-0-12-407693-8.00004-2. Adv Microb Physiol. 2013. PMID: 24054796 Review.
-
Nitric oxide and nitrosative stress tolerance in bacteria.Biochem Soc Trans. 2005 Feb;33(Pt 1):176-80. doi: 10.1042/BST0330176. Biochem Soc Trans. 2005. PMID: 15667299 Review.
Cited by
-
Genome-Wide Identification of Host-Segregating Single-Nucleotide Polymorphisms for Source Attribution of Clinical Campylobacter coli Isolates.Appl Environ Microbiol. 2020 Nov 24;86(24):e01787-20. doi: 10.1128/AEM.01787-20. Print 2020 Nov 24. Appl Environ Microbiol. 2020. PMID: 33036986 Free PMC article.
-
Defining the metabolic requirements for the growth and colonization capacity of Campylobacter jejuni.Front Cell Infect Microbiol. 2014 Sep 29;4:137. doi: 10.3389/fcimb.2014.00137. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25325018 Free PMC article. Review.
-
Strategies of Pathogens to Escape from NO-Based Host Defense.Antioxidants (Basel). 2022 Nov 3;11(11):2176. doi: 10.3390/antiox11112176. Antioxidants (Basel). 2022. PMID: 36358549 Free PMC article. Review.
-
Identification of Campylobacter jejuni genes involved in the response to acidic pH and stomach transit.Appl Environ Microbiol. 2008 Mar;74(5):1583-97. doi: 10.1128/AEM.01507-07. Epub 2008 Jan 11. Appl Environ Microbiol. 2008. PMID: 18192414 Free PMC article.
-
Whole genome sequencing reveals extended natural transformation in Campylobacter impacting diagnostics and the pathogens adaptive potential.Sci Rep. 2020 Feb 28;10(1):3686. doi: 10.1038/s41598-020-60320-y. Sci Rep. 2020. PMID: 32111893 Free PMC article.
References
-
- Bollinger C. J., J. E. Bailey, and P. T. Kallio. 2001. Novel hemoglobins to enhance microaerobic growth and substrate utilization in Escherichia coli. Biotechnol. Prog. 17:798-808. - PubMed
-
- Bonamore, A., A. Farina, M. Gattoni, E. Schininà, A. Bellelli, and A. Boffi. 2003. Interaction with membrane lipids and heme ligand binding properties of Escherichia coli flavohemoglobin. Biochemistry 20:5792-5801. - PubMed
-
- Bonamore, A., P. Gentili, A. Ilari, M. E. Schinina, and A. Boffi. 2003. Escherichia coli flavohemoglobin is an efficient alkylhydroperoxide reductase. J. Biol. Chem. 278:22272-22277. - PubMed
-
- Chen, L., Q. W. Xie, and C. Nathan. 1998. Alkyl hydroperoxide reductase subunit C (AhpC) protects bacterial and human cells against reactive nitrogen intermediates. Mol. Cell 1:795-805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

