Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality
- PMID: 15280480
- PMCID: PMC479089
- DOI: 10.1128/JVI.78.16.8720-8731.2004
Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality
Abstract
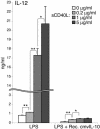
Interleukin-10 (IL-10) suppresses the maturation and cytokine production of dendritic cells (DCs), key regulators of adaptive immunity, and prevents the activation and polarization of naïve T cells towards protective gamma interferon-producing effectors. We hypothesized that human cytomegalovirus (HCMV) utilizes its viral IL-10 homolog (cmvIL-10) to attenuate DC functionality, thereby subverting the efficient induction of antiviral immune responses. RNA and protein analyses demonstrated that the cmvIL-10 gene was expressed with late gene kinetics. Treatment of immature DCs (iDCs) with supernatant from HCMV-infected cultures inhibited both the lipopolysaccharide-induced DC maturation and proinflammatory cytokine production. These inhibitory effects were specifically mediated through the IL-10 receptor and were not observed when DCs were treated with supernatant of cells infected with a cmvIL-10-knockout mutant. Incubation of iDCs with recombinant cmvIL-10 recapitulated the inhibition of maturation. Furthermore, cmvIL-10 had pronounced long-term effects on those DCs that could overcome this inhibition of maturation. It enhanced the migration of mature DCs (mDCs) towards the lymph node homing chemokine but greatly reduced their cytokine production. The inability of mDCs to secrete IL-12 was maintained, even when they were restimulated by the activated T-cell signal CD40 ligand in the absence of cmvIL-10. Importantly, cmvIL-10 potentiates these anti-inflammatory effects, at least partially, by inducing endogenous cellular IL-10 expression in DCs. Collectively, we show that cmvIL-10 causes long-term functional alterations at all stages of DC activation.
Figures




Similar articles
-
Human Cytomegalovirus UL111A and US27 Gene Products Enhance the CXCL12/CXCR4 Signaling Axis via Distinct Mechanisms.J Virol. 2018 Feb 12;92(5):e01981-17. doi: 10.1128/JVI.01981-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237840 Free PMC article.
-
Human Cytomegalovirus-Encoded Human Interleukin-10 (IL-10) Homolog Amplifies Its Immunomodulatory Potential by Upregulating Human IL-10 in Monocytes.J Virol. 2016 Mar 28;90(8):3819-3827. doi: 10.1128/JVI.03066-15. Print 2016 Apr. J Virol. 2016. PMID: 26792743 Free PMC article.
-
Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10.J Immunol. 2004 Sep 1;173(5):3383-91. doi: 10.4049/jimmunol.173.5.3383. J Immunol. 2004. PMID: 15322202
-
Human Cytomegalovirus Interleukin 10 Homologs: Facing the Immune System.Front Cell Infect Microbiol. 2020 Jun 9;10:245. doi: 10.3389/fcimb.2020.00245. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32582563 Free PMC article. Review.
-
Modulation of dendritic cell functions by viral IL-10 encoded by human cytomegalovirus.Front Microbiol. 2014 Jul 4;5:337. doi: 10.3389/fmicb.2014.00337. eCollection 2014. Front Microbiol. 2014. PMID: 25071749 Free PMC article. Review.
Cited by
-
Design and analysis of rhesus cytomegalovirus IL-10 mutants as a model for novel vaccines against human cytomegalovirus.PLoS One. 2011;6(11):e28127. doi: 10.1371/journal.pone.0028127. Epub 2011 Nov 21. PLoS One. 2011. PMID: 22132227 Free PMC article.
-
Impact of Aging and Cytomegalovirus on Immunological Response to Influenza Vaccination and Infection.Front Immunol. 2017 Jul 17;8:784. doi: 10.3389/fimmu.2017.00784. eCollection 2017. Front Immunol. 2017. PMID: 28769922 Free PMC article. Review.
-
Immune activation and regulation in simian immunodeficiency virus-Plasmodium fragile-coinfected rhesus macaques.J Virol. 2013 Sep;87(17):9523-37. doi: 10.1128/JVI.00861-13. Epub 2013 Jun 19. J Virol. 2013. PMID: 23785209 Free PMC article.
-
Attenuation of innate immunity by cytomegalovirus IL-10 establishes a long-term deficit of adaptive antiviral immunity.Proc Natl Acad Sci U S A. 2010 Dec 28;107(52):22647-52. doi: 10.1073/pnas.1013794108. Epub 2010 Dec 13. Proc Natl Acad Sci U S A. 2010. PMID: 21149711 Free PMC article.
-
HCMV IL-10 suppresses cytokine expression in monocytes through inhibition of nuclear factor-kappaB.Viral Immunol. 2008 Dec;21(4):477-82. doi: 10.1089/vim.2008.0048. Viral Immunol. 2008. PMID: 19115937 Free PMC article.
References
-
- Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077-1084. - PubMed
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. - PubMed
-
- Barry, P. A., and W. L. Chang. Primate betaherpesviruses. In A. M. Arvin, G. Campadelli-Fumi, E. S. Mocarski, P. S. Moore, B. Roizman, R. Whitley, and K. Yamanishi (ed.), Human herpesviruses: biology, therapy and immunoprophylaxis, in press. Cambridge University Press, Cambridge, United Kingdom. - PubMed
-
- Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. - PubMed
-
- Bondeson, J., K. A. Browne, F. M. Brennan, B. M. Foxwell, and M. Feldmann. 1999. Selective regulation of cytokine induction by adenoviral gene transfer of IκBα into human macrophages: lipopolysaccharide-induced, but not zymosan-induced, proinflammatory cytokines are inhibited, but IL-10 is nuclear factor-κB independent. J. Immunol. 162:2939-2945. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

