Temporal changes in gene expression after injury in the rat retina
- PMID: 15277499
- PMCID: PMC2821791
- DOI: 10.1167/iovs.03-1047
Temporal changes in gene expression after injury in the rat retina
Abstract
Purpose: The goal of this study was to define the temporal changes in gene expression after retinal injury and to relate these changes to the inflammatory and reactive response. A specific emphasis was placed on the tetraspanin family of proteins and their relationship with markers of reactive gliosis.
Methods: Retinal tears were induced in adult rats by scraping the retina with a needle. After different survival times (4 hours, and 1, 3, 7, and 30 days), the retinas were removed, and mRNA was isolated, prepared, and hybridized to the Affymatrix RG-U34A microarray (Santa Clara, CA). Microarray results were confirmed by using RT-PCR and correlation to protein levels was determined.
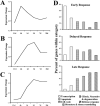
Results: Of the 8750 genes analyzed, approximately 393 (4.5%) were differentially expressed. Clustering analysis revealed three major profiles: (1) The early response was characterized by the upregulation of transcription factors; (2) the delayed response included a high percentage of genes related to cell cycle and cell death; and (3) the late, sustained profile clustered a significant number of genes involved in retinal gliosis. The late, sustained cluster also contained the upregulated crystallin genes. The tetraspanins Cd9, Cd81, and Cd82 were also associated with the late, sustained response.
Conclusions: The use of microarray technology enables definition of complex genetic changes underlying distinct phases of the cellular response to retinal injury. The early response clusters genes associate with the transcriptional regulation of the wound-healing process and cell death. Most of the genes in the late, sustained response appear to be associated with reactive gliosis.
Figures
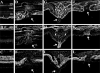

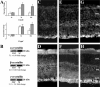
 ) were confirmed with RT-PCR (A, □). Immunoblots show increased levels of crystallin-α, -β, and -γ proteins (B). Crystallin-α, -β, and -γ expression in normal retinas (C, E, G, respectively) and injured retinas 7 days after injury (D, F, H, respectively) was localized by immunohisto-chemistry. In normal retina, crystallin immunoreactivity was present mainly in the GCL (C, E, G). Seven days after injury, there was a strong crystallin immunoreactivity throughout the scar spanning the retinal tear and protruded into the vitreous space (data not shown). High immunoreactivity levels for crystallin-α (D), -β (F), and -γ (H) were found immediately adjacent to the retina at GCL and outer segment layer (OS). No staining was seen when secondary antibody control was used (data not shown). (A) Significant changes from normal: t-test, *P < 0.05, **P < 0.01.
) were confirmed with RT-PCR (A, □). Immunoblots show increased levels of crystallin-α, -β, and -γ proteins (B). Crystallin-α, -β, and -γ expression in normal retinas (C, E, G, respectively) and injured retinas 7 days after injury (D, F, H, respectively) was localized by immunohisto-chemistry. In normal retina, crystallin immunoreactivity was present mainly in the GCL (C, E, G). Seven days after injury, there was a strong crystallin immunoreactivity throughout the scar spanning the retinal tear and protruded into the vitreous space (data not shown). High immunoreactivity levels for crystallin-α (D), -β (F), and -γ (H) were found immediately adjacent to the retina at GCL and outer segment layer (OS). No staining was seen when secondary antibody control was used (data not shown). (A) Significant changes from normal: t-test, *P < 0.05, **P < 0.01.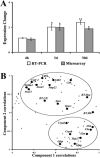
Similar articles
-
Differential temporal and spatial expression of immediate early genes in retinal neurons after ischemia-reperfusion injury.Invest Ophthalmol Vis Sci. 2003 May;44(5):2211-20. doi: 10.1167/iovs.02-0704. Invest Ophthalmol Vis Sci. 2003. PMID: 12714663
-
Genetic networks controlling retinal injury.Mol Vis. 2005 Nov 4;11:958-70. Mol Vis. 2005. PMID: 16288200 Free PMC article.
-
Expression of acute-phase response proteins in retinal Müller cells in diabetes.Invest Ophthalmol Vis Sci. 2005 Jan;46(1):349-57. doi: 10.1167/iovs.04-0860. Invest Ophthalmol Vis Sci. 2005. PMID: 15623795
-
Retinal gene expression and Müller cell responses after branch retinal vein occlusion in the rat.Invest Ophthalmol Vis Sci. 2009 May;50(5):2359-67. doi: 10.1167/iovs.08-2332. Epub 2008 Sep 20. Invest Ophthalmol Vis Sci. 2009. PMID: 18806298
-
Temporal regulation of CD81 following retinal injury in the rat.Neurosci Lett. 2003 Feb 20;338(1):29-32. doi: 10.1016/s0304-3940(02)01364-2. Neurosci Lett. 2003. PMID: 12565133 Free PMC article.
Cited by
-
Intravitreal injection of β-crystallin B2 improves retinal ganglion cell survival in an experimental animal model of glaucoma.PLoS One. 2017 Apr 6;12(4):e0175451. doi: 10.1371/journal.pone.0175451. eCollection 2017. PLoS One. 2017. PMID: 28384305 Free PMC article.
-
Lack of immunoglobulins does not prevent C1q binding to RGC and does not alter the progression of experimental glaucoma.Invest Ophthalmol Vis Sci. 2012 Sep 19;53(10):6370-7. doi: 10.1167/iovs.12-10442. Invest Ophthalmol Vis Sci. 2012. PMID: 22918632 Free PMC article.
-
betaA3/A1-crystallin in astroglial cells regulates retinal vascular remodeling during development.Mol Cell Neurosci. 2008 Jan;37(1):85-95. doi: 10.1016/j.mcn.2007.08.016. Epub 2007 Aug 31. Mol Cell Neurosci. 2008. PMID: 17931883 Free PMC article.
-
Transfer of lens-specific transcripts to retinal RNA samples may underlie observed changes in crystallin-gene transcript levels after ischemia.Mol Vis. 2007 Feb 8;13:220-8. Mol Vis. 2007. PMID: 17327827 Free PMC article.
-
Level of vitreous alpha-B crystallin in eyes with rhegmatogenous retinal detachment.Graefes Arch Clin Exp Ophthalmol. 2015 Aug;253(8):1251-4. doi: 10.1007/s00417-014-2815-z. Epub 2014 Oct 14. Graefes Arch Clin Exp Ophthalmol. 2015. PMID: 25311653
References
-
- Ryan SJ, Stout JT, Dugel PV. Posterior penetrating ocular trauma. In: Ryan SJ, editor. Retina. Vol 3. CV Mosby Co.; St. Louis: 1994. pp. 2235–2250.
-
- Postel EA, Mieler WF. Posterior segment manifestations of blunt trauma. In: Guyer DR, editor. Retina-Vitreous-Macula. Vol 1. WB Saunders Co.; Philadelphia: 1999. pp. 831–843.
-
- Fisher SK, Stone J, Rex TS, Linberg KA, Lewis GP. Experimental retinal detachment: a paradigm for understanding the effects of induced photoreceptor degeneration. Prog Brain Res. 2001;131:679–698. - PubMed
-
- Humphrey MF, Constable IJ, Chu Y, Wiffen S. A quantitative study of the lateral spread of Müller cell responses to retinal lesions in the rabbit. J Comp Neurol. 1993;334:545–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

