Mutations in herpes simplex virus glycoprotein D that prevent cell entry via nectins and alter cell tropism
- PMID: 15273289
- PMCID: PMC515077
- DOI: 10.1073/pnas.0404211101
Mutations in herpes simplex virus glycoprotein D that prevent cell entry via nectins and alter cell tropism
Abstract
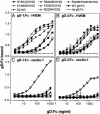
Glycoprotein D (gD) determines which cells can be infected by herpes simplex virus (HSV) by binding to one of the several cell surface receptors that can mediate HSV entry or cell fusion. These receptors include the herpesvirus entry mediator (HVEM), nectin-1, nectin-2, and sites in heparan sulfate generated by specific 3-O-sulfotransferases. The objective of the present study was to identify residues in gD that are critical for physical and functional interactions with nectin-1 and nectin-2. We found that double or triple amino acid substitutions at positions 215, 222, and 223 in gD caused marked reduction in gD binding to nectin-1 and a corresponding inability to function in cell fusion or entry of HSV via nectin-1 or nectin-2. These substitutions either enhanced or did not significantly inhibit functional interactions with HVEM and modified heparan sulfate. These and other results demonstrate that different domains of gD, with some overlap, are critical for functional interactions with each class of entry receptor. Viral entry assays, using gD mutants described here and previously, revealed that nectins are the principal entry receptors for selected human cell lines of neuronal and epithelial origin, whereas HVEM or nectins could be used to mediate entry into a T lymphocyte line. Because T cells and fibroblasts can be infected via HVEM, HSV strains carrying gD mutations that prevent entry via nectins may establish transient infections in humans, but perhaps not latent infections of neurons, and are therefore candidates for development of safe live virus vaccines and vaccine vectors.
Figures
Similar articles
-
Mutations in the N termini of herpes simplex virus type 1 and 2 gDs alter functional interactions with the entry/fusion receptors HVEM, nectin-2, and 3-O-sulfated heparan sulfate but not with nectin-1.J Virol. 2003 Sep;77(17):9221-31. doi: 10.1128/jvi.77.17.9221-9231.2003. J Virol. 2003. PMID: 12915538 Free PMC article.
-
B Virus (Macacine Herpesvirus 1) Divergence: Variations in Glycoprotein D from Clinical and Laboratory Isolates Diversify Virus Entry Strategies.J Virol. 2016 Sep 29;90(20):9420-32. doi: 10.1128/JVI.00799-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27512063 Free PMC article.
-
Effects of linker-insertion mutations in herpes simplex virus 1 gD on glycoprotein-induced fusion with cells expressing HVEM or nectin-1.Virology. 2004 Jan 5;318(1):318-26. doi: 10.1016/j.virol.2003.10.004. Virology. 2004. PMID: 14972557
-
Two Sides to Every Story: Herpes Simplex Type-1 Viral Glycoproteins gB, gD, gH/gL, gK, and Cellular Receptors Function as Key Players in Membrane Fusion.Viruses. 2021 Sep 16;13(9):1849. doi: 10.3390/v13091849. Viruses. 2021. PMID: 34578430 Free PMC article. Review.
-
The structural basis of herpesvirus entry.Nat Rev Microbiol. 2021 Feb;19(2):110-121. doi: 10.1038/s41579-020-00448-w. Epub 2020 Oct 21. Nat Rev Microbiol. 2021. PMID: 33087881 Free PMC article. Review.
Cited by
-
Bioprocess development for biosurfactant production by Natrialba sp. M6 with effective direct virucidal and anti-replicative potential against HCV and HSV.Sci Rep. 2022 Oct 4;12(1):16577. doi: 10.1038/s41598-022-20091-0. Sci Rep. 2022. PMID: 36195643 Free PMC article.
-
Bispecific adapter-mediated retargeting of a receptor-restricted HSV-1 vector to CEA-bearing tumor cells.Mol Ther. 2011 Mar;19(3):507-14. doi: 10.1038/mt.2010.207. Epub 2010 Oct 5. Mol Ther. 2011. PMID: 20924362 Free PMC article.
-
Restoring Herpesvirus Entry Mediator (HVEM) Immune Function in HVEM-/- Mice Rescues Herpes Simplex Virus 1 Latency and Reactivation Independently of Binding to Glycoprotein D.J Virol. 2020 Jul 30;94(16):e00700-20. doi: 10.1128/JVI.00700-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32522859 Free PMC article.
-
Mapping sites of herpes simplex virus type 1 glycoprotein D that permit insertions and impact gD and gB receptors usage.Sci Rep. 2017 Mar 3;7:43712. doi: 10.1038/srep43712. Sci Rep. 2017. PMID: 28255168 Free PMC article.
-
Harnessing the Potential of Biosurfactants for Biomedical and Pharmaceutical Applications.Pharmaceutics. 2023 Aug 18;15(8):2156. doi: 10.3390/pharmaceutics15082156. Pharmaceutics. 2023. PMID: 37631370 Free PMC article. Review.
References
-
- Spear, P. G., Eisenberg, R. J. & Cohen, G. H. (2000) Virology 275, 1–8. - PubMed
-
- Montgomery, R. I., Warner, M. S., Lum, B. J. & Spear, P. G. (1996) Cell 87, 427–436. - PubMed
-
- Granger, S. W. & Rickert, S. (2003) Cytokine Growth Factor Rev. 14, 289–296. - PubMed
-
- Geraghty, R. J., Krummenacher, C., Cohen, G. H., Eisenberg, R. J. & Spear, P. G. (1998) Science 280, 1618–1620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

